01 January 2021: Articles 
Superficial Cerebral Venous Thrombosis and Intracerebral Hematoma in a 48-Year-Old Man with SARS-CoV-2 Infection: A Case Report
Unusual clinical course, Challenging differential diagnosis, Unusual setting of medical care, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Erico Ramos Cardoso1ADE*, Sandeep Singh Bains1BEF, Benjamin Robison1EF, Jeffrey Farkas2EDOI: 10.12659/AJCR.927011
Am J Case Rep 2021; 22:e927011
Abstract
BACKGROUND: Pandemic coronavirus disease 2019 (COVID-19) originated in Wuhan, China, and is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Severe respiratory symptoms are a hallmark of the disease, which may also include complications related to a hypercoagulable state and central nervous system involvement. These complications can occur during either the acute or the recovery phase. The cerebral involvement typically manifests as intracranial hypertension, intracerebral hemorrhage, diffuse encephalopathy, or cerebral venous thrombosis. The hemorrhagic form of cerebral venous thrombosis can be a diagnostic challenge and is treated by anticoagulation therapy, despite the existence of an intracerebral hemorrhage. This report describes a case of superficial cerebral venous thrombosis and intracerebral hematoma in a 48-year-old man weeks after recovering from the acute phase of SARSCoV-2 infection.
CASE REPORT: A 48-year-old man with a past medical history of SARS-CoV-2 infection confirmed by SARS-CoV-2 reverse-transcription polymerase chain reaction presented with left upper-limb numbness, weakness, and impaired positional sensorium. After initial stabilization, noncontrast computerized tomography and magnetic resonance imaging confirmed an intracerebral hemorrhage with underlying cerebral venous thrombosis. The patient was successfully treated with enoxaparin anticoagulation therapy, and symptoms improved over the following 12 days.
CONCLUSIONS: Central nervous system venous thrombosis is an atypical presentation of the hypercoagulable state primarily seen in younger patients, and it can occur in a delayed fashion after recovery from mild forms of COVID-19.
Keywords: Cerebral Hemorrhage, COVID-19, Embolism and Thrombosis, Intracranial Thrombosis, Anticoagulants, COVID-19, enoxaparin, Hematoma, Magnetic Resonance Imaging, Tomography, X-Ray Computed, Venous Thrombosis
Background
Human infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which originated in Wuhan, China, can present with multiple clinical manifestations. The most common presentation, coronavirus disease 2019 (COVID-19), involves respiratory symptoms. In several cases, the clinical manifestations have been dominated by complications caused by systemic inflammation and a hypercoagulable state. In the central nervous system (CNS), such complications can manifest in the form of an altered mental state, hallucinations, and either ischemic or hemorrhagic strokes [1]. Neurological manifestations are believed to originate from SARS-CoV-2 entering the CNS through the olfactory bulb or by thrombosis of the cerebral vasculature, secondary to hypercoagulability. Direct invasion of the CNS by the virus results in demyelination and inflammation [2]. In the presence of SARS-CoV-2, glial cells secrete acute-phase reactants such as interleukin-6, interleukin-12p40, interleukin-15, tumor necrosis factor-alpha, chemokine ligand 9, and chemokine ligand 10 [2]. Furthermore, SARS-CoV-2 is hypothesized to utilize the angiotensin-converting enzyme 2 receptor (which is expressed on glial cells and neurons) to permeate CNS cells, causing inflammation and damage to surrounding structures [2]. Cerebral venous thromboembolism, with or without associated intracerebral hemorrhage, is much less frequent [3]. The underlying mechanism for the development of hypercoagulability and subsequent stroke in patients with COVID-19 is believed to be similar to the patho-physiology of more common systemic coagulopathies, such as disseminated intravascular coagulation or thrombotic microangiopathy [4]. However, the management of the hypercoagulable state and acute stroke in the setting of a pandemic poses unique challenges. These include the need to perform stroke management and interventions while dealing with the prevention of nosocomial spread of infection [5]. This report describes a case of superficial cerebral venous thrombosis and intracerebral hematoma in a 48-year-old man weeks after recovering from the acute phase of SARSCoV-2 infection.
Case Report
A 48-year-old right-handed man, who was a nonsmoker with a past medical history of SARS-CoV-2, presented with sudden onset of left upper-limb numbness, weakness, and impairment of positional sensation. He experienced severe right occipital headaches for 8 h, for which he took ibuprofen. Thirty-six days previously he had presented with fever, malaise, low-back pain, and right-sided headaches. An RNA polymerase chain reaction test was positive for COVID-19, and the diagnosis was confirmed via nasopharyngeal swab, using the SARS-CoV-2 reverse-transcription polymerase chain reaction test. His symptoms lasted for 12 days, during which he quarantined at home. He returned to work on day 17.
On admission for stroke symptomatology, the patient was hypertensive at 178/89 mmHg and afebrile. His O2 saturation was 98% on room air, his heart rate was 88 beats/min, and his respiratory rate was 17 breaths/min. The patient had a body mass index of 27.4 kg/m2. He was fully alert and oriented to person, place, and time, and he was behaving appropriately. Motor testing demonstrated 3/5 strength of all muscle groups of the left upper limb. There was cerebellar dysmetria of the left arm. The National Institutes of Health Stroke Scale score was 4. The abnormal/pertinent laboratory findings are listed in the Table 1. Thrombophilia workup included a minimally high factor 8 activity at 162% (range 50–149%); lupus anticoagulant was negative, and beta 2 glycoprotein and cardiolipin antibodies were normal. Other causes of cerebral venous thrombosis were ruled out.
Noncontrast head computed tomography (CT) showed a small acute cortical hemorrhage in the right parietal lobe, with a rim of surrounding vasogenic edema (Figure 1A). On the serial axial images, starting at the hemorrhage and progressing to the superior sagittal sinus, there was a curvilinear structure of increased attenuation that overlaid the cortex. The higher-intensity CT signal indicated clotted blood within a thrombosed cortical vein (Figure 2). CT angiography of neck and head was normal, with no vascular malformation. Magnetic resonance imaging of the head confirmed the presence of a right parietal lesion (Figure 1B). The magnetic resonance venography was diagnostic of a cerebral venous thrombosis. It showed an absent right parietal vein as well as a small filling defect where the vein entered the superior sagittal sinus (Figure 3). The blood pressure was controlled at 147/87 mmHg by using 20 mg of intravenous labetalol. While admitted, the patient was given 80 mg of subcutaneous enoxaparin every 12 h for a total of 2 doses and 500 mg of intravenous levetiracetam for a total of 2 doses. The patient remained neurologically stable throughout the admission. After discharge, he progressed satisfactorily, with slow improvement of symptoms and signs. He returned to work a week after discharge while undergoing occupational therapy. At 4 and 8 weeks after the stroke, the only residual deficit was a mild numbness of the left pointer finger. Follow-up magnetic resonance imaging demonstrated clot maturation and progressive shrinkage of the intracerebral hematoma (Figure 4).
Discussion
In this case study, we report the hemostatic manifestations and thrombotic complications (stroke) experienced by a 48-year-old man, in association with COVID-19 due to SARS-CoV-2 infection. The pathophysiology of the hypercoagulable state in SARS-CoV-2 infection is multifactorial: it involves (i) an increase in acute-phase reactants (cytokine storm), (ii) endothelial damage sustained due to SARS-CoV-2, and (iii) diffuse microvascular injury with resulting thrombosis [6]. These findings are supported in a study by Md Noh [7], who found that patients with COVID-19 show prolonged prothrombin time, as well as increased D-dimer levels and low-normal platelet counts. While thromboembolic complications are relatively uncommon in COVID-19, it is important to keep them in mind because of their potential severity and the diversity of clinical presentations. Delayed diagnosis may lead to complications such as progression of thrombosis into the cerebral venous sinuses, intracerebral hemorrhage, and intracranial hypertension. If diagnosed prior to the onset of complications, the prognosis of cerebral venous thrombosis is favorable in the majority of patients [8,9]. Girot et al. [10] reported 6 cases of cerebral venous thrombosis complications in COVID-19 patients. The patients’ symptoms were apparent at initial presentation or manifested within 15 days from the onset of COVID-19, and 3 of the patients had an associated parenchymal hemorrhage.
Information is available for 5 of the 6 patients: 2 were men and 3 were women. The mean age was 56.8±7 years, and these patients were therefore older than our patient [10]. The relatively young age of our patient may have contributed to his early favorable prognosis (modified Rankin scale score of 1 at 4 weeks after his stroke). In contrast, non-COVID-19 patients who present with hemorrhagic forms of cerebral venous thrombosis tend to have a poorer prognosis [11].
It is important to differentiate the cause of the intracerebral hemorrhage in COVID-19 patients. Primary intracranial hemorrhage, unrelated to venous cerebral thrombosis, is a better-known neurological complication during the acute manifestation of COVID-19. Obviously, anticoagulation is not considered in these patients. Benger et al. [12] reported 5 such cases of intracranial hemorrhage associated with the active phase of COVID-19. These cases occurred in patients who were younger than the average age of non-COVID-19 patients with intracranial hemorrhage. The complication occurred in patients with prolonged inflammatory syndrome and was attributed to the inflammatory endotheliopathy caused by COVID-19. None of their patients had cerebral vein thrombosis. Dogra et al. [13] reported 33 such patients, who developed intracranial hemorrhage during acute treatment for COVID-19. They attributed the complication to the administration of anticoagulants used to avoid thrombotic complications of COVID-19.
A less frequent cerebrovascular complication of COVID-19 is cerebral vein thrombosis, with or without associated hemorrhagic venous infarct, which occurred in the current case. The hemorrhagic cases have some features in common, including young age, initial presentation of deep vein thrombosis symptoms, and milder forms of COVID-19 only discovered after the stroke, within less than 2 weeks apart [14]. Cavalcanti et al. [15] described 3 patients younger than 41 years who presented with cerebral vein thrombosis within 1 week of developing COVID-19 symptoms. Hemasian and Ansari [16] reported a 65-year-old man with a hemorrhagic form of cerebral venous thrombosis as the first manifestation of COVID-19. Similarly, Klein et al. [17] reported a 29-year-old patient with hemorrhagic cerebral vein thrombosis also presenting within the first week of COVID-19. Roy-Gash et al. [18] described a 63-year-old woman who presented with right hemiplegia from a hemorrhagic cerebral vein thrombosis 12 days after onset of COVID-19 symptoms. While our patient shared the common features of younger age and mild presentation of COVID-19, the interval between onset of COVID-19 symptoms and cerebral vein thrombosis of 26 days was much longer than any of the cases previously discussed. This raises the possibility that the hypercoagulable state associated with COVID-19 outlasts the active phase of the infectious disease.
In a study by Silvis et al. [19], 4 main presentations of cerebral venous thrombosis were described, including isolated intracranial hypertension, focal neurological deficits, diffuse encephalopathy, and cavernous sinus syndrome. Heparin anticoagulation should be started immediately in all presentations to decrease levels of cytokines prophylactically, even if patients present with a secondary intracerebral hemorrhage, as was the case with our patient [20]. Despite recorded success of endovascular thrombolysis, a recent trial by Coutinho et al. [21] supports decreased morbidity with the traditional systemic use of intravenous heparin therapy alone. This finding is in agreement with the favorable prognosis in our patient, who returned to full employment after a 2-week quarantine with COVID-19. Twenty-six days later he developed the hemorrhagic form of cerebral venous thrombosis symptoms, and he returned to work 1 week after hospital discharge. Furthermore, the lag of 26 days between onset of symptomatic COVID-19 and stroke in our patient is considerably longer than the interval previously reported. It raises the question of whether the prophylactic use of antiplatelet or anticoagulant therapy should be considered for patients recovering from milder forms of COVID-19.
Conclusions
This case of superficial cerebral venous thrombosis and intracerebral hematoma in a 48-year-old man with SARS-CoV-2 infection has shown that even mild cases of COVID-19 may be associated with coagulopathy and an increased risk of cerebral thrombosis and hemorrhage. As clinical management guidelines continue to evolve, care should be taken to provide the most appropriate and timely individualized anticoagulation therapy in patients with SARS-CoV-2 infection who present with neurological signs and symptoms.
Figures
References:
1.. Md Noh MSF, COVID-19 and cerebral hemorrhage: Proposed mechanisms: J Neuroradiol, 2020 [Online ahead of print]
2.. Tsai ST, Lu MK, San S, Tsai CH, The neurologic manifestations of coronavirus disease 2019 pandemic: A systematic review: Front Neurol, 2020; 11; 498
3.. Qureshi A, Abd-Allah F, Al-Senani F, Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel: Int J Stroke, 2020; 15(5); 540-54
4.. Levi M, Thachil J, Iba T, Levy JH, Coagulation abnormalities and thrombosis in patients with COVID-19: Lancet Haematol, 2020; 7(6); e438-40
5.. Morassi M, Bagatto D, Cobelli M, Stroke in patients with SARS-CoV-2 infection: Case series: J Neurol, 2020; 267(8); 2185-92
6.. Palumbo D, Guazzarotti G, De Cobelli F, Spontaneous major hemorrhage in COVID-19 patients: Another brick in the wall of SARS-CoV-2 – associated coagulation disorders?: J Vasc Interv Radiol, 2020; 31(9); 1494-96
7.. Md Noh MSF, Brain imaging findings in COVID-19: What do we know so far?: J Neuroradiol, 2020; 47(5); 329-30
8.. Dentali F, Poli D, Scoditti U, Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study: J Thromb Haemost, 2012; 10(7); 1297-302
9.. Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T, Long-term outcome after cerebral venous thrombosis: Analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients: J Neurol, 2016; 263(3); 477-84
10.. Girot M, Ferro J, Canhão P, Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage: Stroke, 2007; 38(2); 337-42
11.. Hughes C, Nichols T, Pike M, Cerebral venous sinus thrombosis as a presentation of COVID-19: Eur J Case Rep Intern Med, 2020; 7(5); 001691
12.. Benger M, Williams O, Siddiqui J, Sztriha L, Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series: Brain Behav Immun, 2020; 88; 940-44
13.. Dogra S, Jain R, Cao M, Hemorrhagic stroke and anticoagulation in COVID-19: J Stroke Cerebrovasc Dis, 2020; 29(8); 104984
14.. Garaci F, Di Giuliano F, Picchi E, Venous cerebral thrombosis in COVID-19 patient: J Neurol Sci, 2020; 414; 116871
15.. Cavalcanti D, Raz E, Shapiro M, Cerebral venous thrombosis associated with COVID-19: Am J Neuroradiol, 2020; 41(8); 1370-76
16.. Hemasian H, Ansari B, First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case: Rev Neurol (Paris), 2020; 176(6); 521-23
17.. Klein D, Libman R, Kirsch C, Arora R, Cerebral venous thrombosis: Atypical presentation of COVID-19 in the young: J Stroke Cerebrovasc Dis, 2020; 29(8); 104989
18.. Roy-Gash F, De Mesmay M, Devys J, COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features: Crit Care, 2020; 24(1); 419
19.. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM, Cerebral venous thrombosis: Nat Rev Neurol, 2017; 13(9); 555-65
20.. Miesbach W, Makris M, COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation: Clin Appl Thromb Hemost, 2020; 26; 1076029620938149
21.. Coutinho J, Zuurbier S, Bousser M: JAMA Neurol, 2020; 77(8); 966-73
Figures
Tables
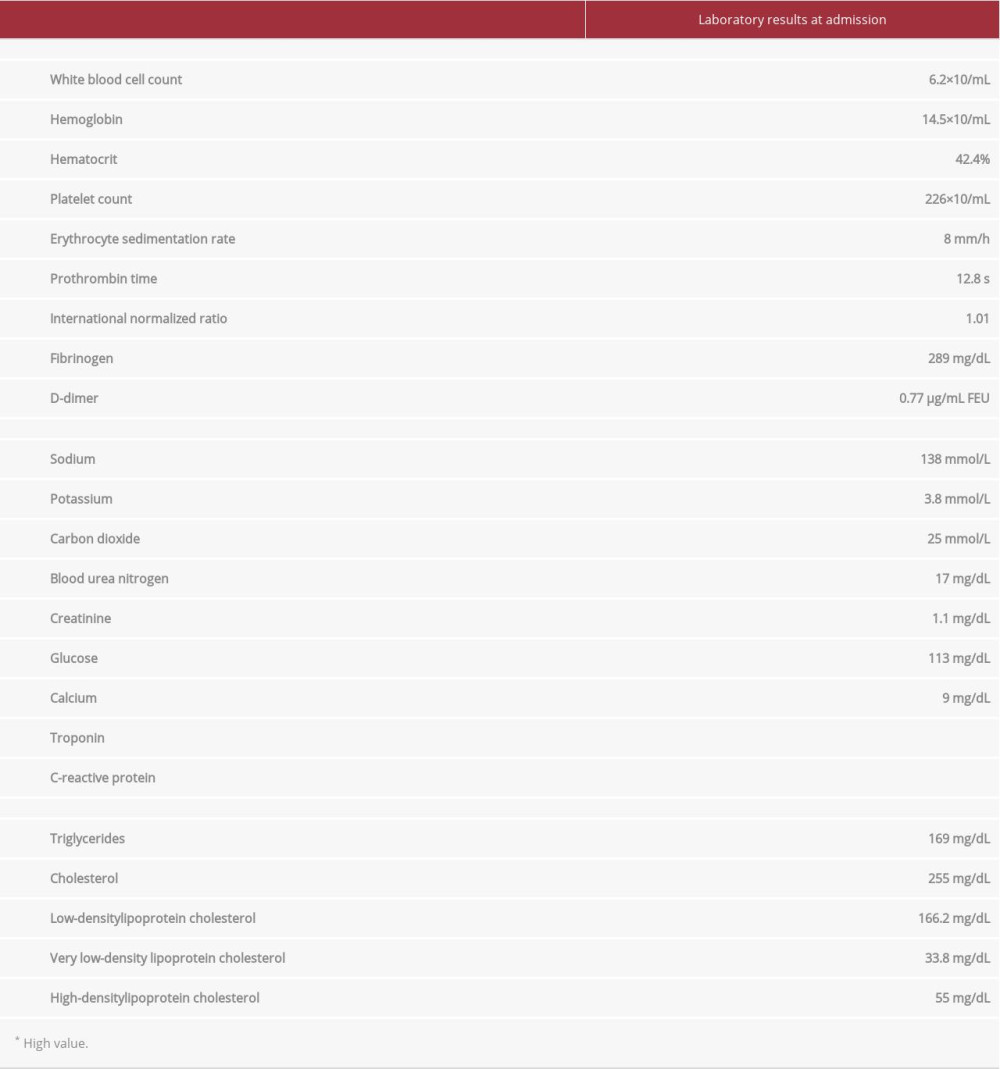 Table 1.. The laboratory findings on admission to hospital of a 48-year old man with severe acute respiratory syndrome coronavirus 2 infection and superficial cerebral venous thrombosis and subdural hematoma.
Table 1.. The laboratory findings on admission to hospital of a 48-year old man with severe acute respiratory syndrome coronavirus 2 infection and superficial cerebral venous thrombosis and subdural hematoma. Table 1.. The laboratory findings on admission to hospital of a 48-year old man with severe acute respiratory syndrome coronavirus 2 infection and superficial cerebral venous thrombosis and subdural hematoma.
Table 1.. The laboratory findings on admission to hospital of a 48-year old man with severe acute respiratory syndrome coronavirus 2 infection and superficial cerebral venous thrombosis and subdural hematoma. In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








