08 April 2021: Articles 
A 58-Year-Old Woman with Acute Gastric Perforation Due to Metastatic Ductal Carcinoma 18 Years Following Bilateral Mastectomy for Invasive Ductal Carcinoma of the Breast
Unusual clinical course, Challenging differential diagnosis, Diagnostic / therapeutic accidents, Unusual setting of medical care, Educational Purpose (only if useful for a systematic review or synthesis)
William A. Nehmeh1ABDEF*, Joseph Derienne1ABCDE, Léa El Khoury2BD, Serge Kassar1BD, Viviane Track-Smayra2BD, Roger Noun1AF, Ghassan Chakhtoura1AEFDOI: 10.12659/AJCR.927094
Am J Case Rep 2021; 22:e927094
Abstract
BACKGROUND: Invasive lobular carcinoma and ductal carcinoma of the breast can metastasize to all sites in the body, including the gastrointestinal tract. Late presentation of metastases of lobular carcinoma of the breast to the gastrointestinal tract have previously been reported, but late metastasis of ductal carcinoma of the breast to the gastric mucosa is rare. This report is of a 58-year-old Lebanese woman who presented with acute gastric perforation due to metastatic ductal carcinoma,18 years following bilateral mastectomy for invasive ductal carcinoma of the breast.
CASE REPORT: We present the case of a 58-year-old woman who underwent a right modified mastectomy for an invasive ductal carcinoma in 2002 combined with a contralateral prophylactic mastectomy for cosmetic purposes. She presented a secondary gastric lesion 18 years later. The clinical presentation resembled perforated ulcer. The choice of gastrectomy was denied due to retrogastric and pancreatic invasion by the tumor. A laparoscopic gastric closure failed to heal the perforation. A supraumbilical laparotomy incision was performed for the placement of a Pezzer tube in the gastric perforation and the installation of a feeding jejunostomy.
CONCLUSIONS: This report is of a rare presentation of metastatic ductal carcinoma of the breast to the gastric mucosa associated with gastric perforation that presented 18 years after bilateral mastectomy. This case highlights the importance of obtaining a full past medical history to identify previous primary malignancy, and also is a reminder that ductal carcinoma of the breast can present with metastatic involvement in the gastrointestinal tract several months, or even years, following mastectomy.
Keywords: Breast Neoplasms, Digestive System Diseases, Breast, Carcinoma, Ductal, Breast, Carcinoma, Lobular, Mastectomy
Background
Breast cancer is the most common cancer in the world and represents the fifth most common cause of death among women. It accounted for 30% of all cancers in 2020 [1]. The lifetime risk of an American woman developing breast cancer is 12.5% [2].
About 12% of patients diagnosed with breast cancer will develop metastatic disease, mainly in the lungs, the liver, the bones, and the brain [3,4]. However, digestive tract metastasis of breast carcinoma is rare, with the stomach being the second most frequent gastrointestinal location after the colon [5]. The invasive lobular carcinoma (ILC) subtype is most commonly responsible of gastric metastasis [6], with a molecular pattern dominated by estrogen receptor positive (ER+) and human epidermal growth factor 2 (HER2) negative sub-types. In 2017, Mathew et al reported on the distinct patterns of metastases from invasive lobular carcinoma of the breast and showed that, when compared with invasive ductal carcinoma, patients with invasive lobular carcinoma were more likely to develop metastases to the ovary and gastrointestinal tract [7]. Differentiating gastric primary and secondary lesions is a crucial challenge, and relies on clinical, endoscopic, and pathological features with different therapeutic implications [8]. Gastrointestinal metastasis of breast cancer is rarely diagnosed, with most cases being either asymptomatic, or synchronous to other metastatic sites upon diagnosis, or even discovered on autopsies [5,9]. Recently, De Gruttola et al reported the case of a 61-year-old woman who presented with gastric perforation due to metastatic lobular carcinoma of the breast, 8 years following diagnosis and mastectomy [10]. The present report is of a 58-year-old Lebanese woman who presented with acute gastric perforation due to metastatic ductal carcinoma of the breast 18 years following bilateral mastectomy.
Case Report
A 58-year-old woman presented to the emergency department with severe acute abdominal pain of 4 hours’ duration with tachycardia and fever. On physical examination, she had diffuse abdominal tenderness with abdominal guarding in the epigastric area. The pain was not relieved by morphine injections. The medical history revealed dyspepsia, mild gastroesophageal reflux during the previous 6 months, with no hematemesis, nor melena, nor hematochezia. The patient was not taking any regular medication. Surgical history included a right modified mastectomy for an invasive ductal carcinoma (IDC), 18 years previously, combined with contralateral mastectomy for cosmetic purposes. Laboratory tests identified a white blood count of 9400 with 80% neutrophils and elevated C-reactive protein without any other abnormalities. Patient serum albumin level was 3.2 g/L and the patient had significant weight loss during the previous 2 months. Computed tomography scan with intravenous injection showed a gastric perforation with diffuse pneumoperitoneum. It also revealed a tumor invading the posterior stomach wall, the celiac trunk, the splenic vein, and the left pancreas. A dilated Wirsung canal was also noted. An infiltrative gastric or pancreatic tumor was suspected (Figures 1, 2).
Laparoscopic exploration of the abdominal cavity showed diffuse peritonitis. A perforation of 2 cm diameter was identified in the anterior wall of the stomach at the level of the incisura angularis. The gastric wall surrounding the perforation area was thick and friable. The tumor bulk had invaded the lesser sac and the pancreas. An initial peritoneal lavage with normal saline solution followed by a biopsy of the perforation margins was performed. We then performed a laparoscopic simple interrupted suture of the perforation with absorbable synthetic 3-0 filament. Two large drains were placed along the sutures. The patient was given parenteral nutrition for 10 days and received 40 mg of omeprazole twice daily. An ingested computed tomography (CT) scan showed no gastric leakage on day 10. The patient was discharged after tolerating an enteral liquid diet on postoperative day 15. Histopathology of the tumor from the site of gastric perforation showed infiltrating malignant cells with histological characteristics of ductal carcinoma, which was supported by positive immunostaining for cytokeratin 7 (CK7) and GATA3, and negative immunostaining for estrogen receptor (ER) and HER2 (Figure 3). This result is compatible with the patient’s breast primary IDC. A switch of the hormonal status was noted since the origin pathology was an ER+ tumor.
The initial breast surgery was a right modified mastectomy performed in March 2002. TNM staging was T2N1M0. The patient received adjuvant chemotherapy, which consisted of 4 cycles of taxotere and 4 cycles of endoxan followed by 5 years of prophylactic selective estrogen receptor modulators (SERMs). She stayed in full remission until the gastric perforation revealed gastric metastasis 214 months later.
One week after hospital discharge, the patient was readmitted for acute abdominal pain. CT scan revealed a gastric leak from the sutured perforation. A mini supraumbilical laparotomy incision was performed for the placement of a Pezzer tube in the gastric perforation and the installation of a feeding jejunostomy. Exploration confirmed that the tumor was not resectable due to multiple-organ involvement. The patient was discharged on postoperative day 12 after tolerating enteral nutrition. The Pezzer tube was kept in place. Two months after her discharge, the patient was doing well, with a plan to start immunotherapy with pembrolizumab.
Discussion
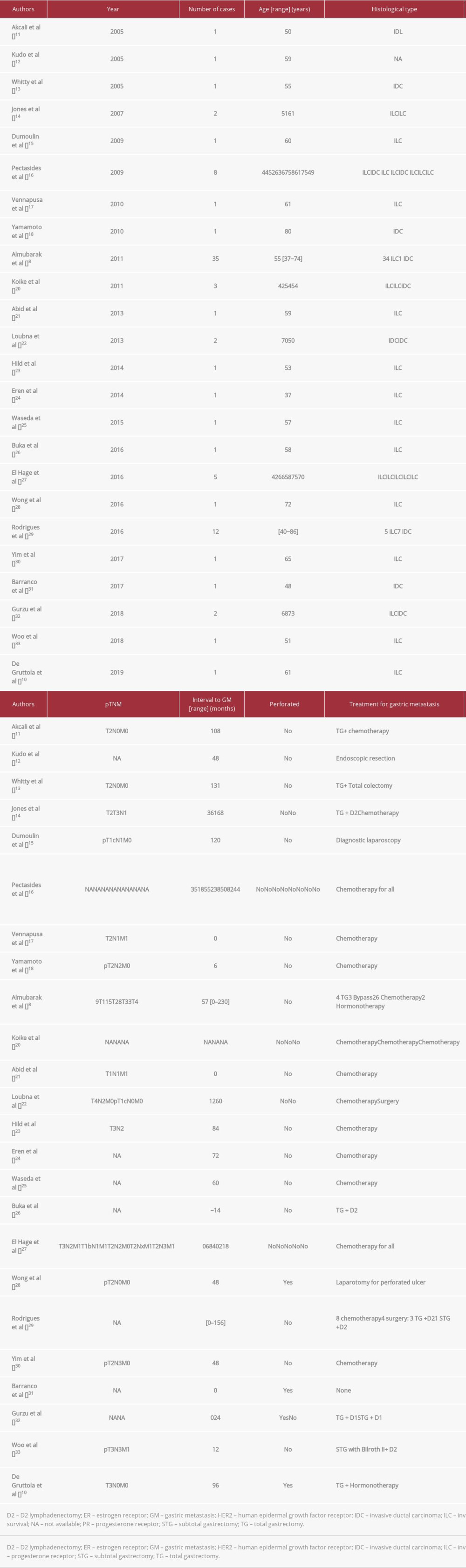
Asch et al provided the first description of metastasis of breast cancer to the gastrointestinal tract from autopsies in 1968; such metastasis was found in 25% of the 52 reported cases [11]. The largest retrospective series studying the spectrum of gastrointestinal metastasis of breast carcinoma reported an incidence of 0.3% for gastric metastasis [6]. Table 1 summarizes the findings from previously reported cases of metastatic ductal and lobular breast carcinoma involving the stomach [8,10,12–33].
Our patient’s clinical presentation, which mimicked a perfo-rated ulcer, is a very rare clinical presentation of the disease, found only in 4 cases in the literature [10,28,31,32].
The primary breast cancer histological subtype in our patient was IDC, which represents only 20% of breast carcinomas responsible for gastric metastasis; ILC represents the remaining 80% [9].
The ability of primary tumors to spread and form secondary lesions differs between ILC and IDC tumors. The loss of expression of E-cadherin, a cell-to-cell adhesion molecule characteristic of ILC tumors, is found in 90% of metastatic cases [34] and may be responsible for the aggressive spread of ILC [35,36]. Other authors have signaled that gastrointestinal tract metastasis mechanisms are more complicated and are still poorly understood for both subtypes [37].
The diagnosis of gastric breast cancer metastasis is always difficult, both clinically and histologically. Clinical symptoms like anorexia, weight loss, epigastric pain, nausea, and vomiting are unspecific. This could cause an underestimation of the prevalence of this condition and a delay in its diagnosis [8]. Histological differentiation between breast cancer gastric metastasis and diffuse gastric primary carcinoma, also called signet cell carcinoma, is difficult [37]. Furthermore, the metastatic lesions are often misdiagnosed as signet cell carcinoma on biopsies. Increased expression of estrogen receptor on immunohistochemistry can differentiate between the two. Other useful markers include gross cystic breast disease fluid protein (GCDFP-15) and cytokeratin (CK) 5/6, especially when the tumor is ER negative [38]. In our case, confirmation of the secondary origin of the gastric biopsy was challenging due to the switch in ER status from positive in the primary breast cancer to negative in the secondary gastric biopsy. This switch is frequently noted in gastric breast cancer metastasis [39]. Our patient’s primary tumor was HER2 positive but positivity for the HER2 receptor cannot confirm a breast origin because 17.9% of gastric adenocarcinomas express this receptor [40]. The HER2 positivity rate in ILC cases, according to Almubarak et al [8], is 9%, and in our review, we found a very similar rate of 8.8%. The connection between HER2 and ER status, and metastasis of breast cancer to the gastrointestinal tract in the IDC subtype, has not been properly studied, and needs further investigation. In our case, CK7 positivity confirmed the tumor’s adenocarcinomal nature [41], while the GATA3 positive state confirmed its mammary origin; this is more accurate than GCDFP-15 [42].
The mean interval between primary breast cancer and gastrointestinal metastasis diagnosis is around 7 years or 84 months [5]. As shown in Table 1, the longest reported interval is 230 months after breast cancer diagnosis [8]. Our patient presents the second longest interval between a primary breast cancer diagnosis and the discovery of a secondary gastric lesion, and the longest interval for an IDC subtype tumor. The interval was 214 months. The relapse after many years following primary diagnosis and adequate initial therapy can be explained by latent micro-metastases resistant to anti-ER therapy, such as the 5 years of treatment with SERM undergone by our patient. Some authors suggest that a delay in disease recurrence may be due to the addition of anti-aromatase medication for another 5 years without clearing the risk of metastatic clones. These hypotheses may explain the long time interval between initial diagnosis and the onset of gastric disease [43,44].
The role of radical resection is limited in treating breast cancer gastric metastasis, and palliative surgery has shown no effect on overall survival [5]. Synchronous extra-digestive metastases are frequently found at the time of gastrointestinal breast cancer metastasis diagnosis, and serve as supporting evidence of a large systemic dissemination of the disease. Systemic therapy such as chemotherapy or hormonotherapy are considered in such cases rather than surgical resection [37]. In rare cases, aggressive surgical treatment has been recommended for a solitary gastric metastasis, followed by chemotherapy and systemic treatment; such courses of treatment have shown minor benefit to overall survival [45]. Less invasive techniques should be considered, such as embolization for gastric bleeding and endoscopic stent placement for obstruction [46,47]. In general, the median survival of patients with metastatic breast cancer ranges between 2 and 3 years [48]. In our review, rapid death was reported in 1 case [31], and the longest reported survival was 66 months [33]. Our patient is still alive at the time of writing of this paper, 3 months after the discovery of her gastric metastasis.
In our case, the patient had gastric perforation with diffuse peritonitis. To our knowledge, we are the first team to attempt a laparoscopic primary closure of the perforation, taking a less invasive approach without knowing the initial diagnosis at the time of surgery. Prior surgical approaches include aggressive total gastrectomy (with no mention of patient survival benefit) [10], and a laparotomy for lavage and drainage [12]. Understanding the unresectable status of the tumor, our second intervention consisted of a mini supraumbilical incision for the placement of a Pezzer tube in the gastric perforation and the installation of a feeding jejunostomy. This approach was chosen to facilitate further systemic treatment and provide appropriate nutritional support, as mortality is associated with malnutrition in advanced metastatic disease [47,48].
Conclusions
This report is of a rare presentation of metastasis of ductal carcinoma of the breast to the gastric mucosa, associated with gastric perforation, presenting 18 years after bilateral mastectomy. This case highlights the importance of obtaining a full past medical history to identify previous primary malignancy, and also is a reminder that carcinoma of the breast can present with metastatic involvement of the gastrointestinal tract several months, or even years, following mastectomy.
Figures
References:
1.. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020: Cancer J Clin, 2020; 70; 7-30
2.. DeSantis C, Ma J, Bryan L, Jemal A, Breast cancer statistics, 2013: Cancer J Clin, 2014; 64; 52-62
3.. Peart O, Metastatic breast cancer: Radiol Technol, 2017; 88; 519M-39M
4.. Arslan D, Tural D, Tatlı AM, Isolated uterine metastasis of invasive ductal carcinoma: Case Rep Oncol Med, 2013; 2013; 793418
5.. McLemore EC, Pockaj BA, Reynolds C, Breast cancer: Presentation and intervention in women with gastrointestinal metastasis and carcinomatosis: Ann Surg Oncol, 2005; 12; 886-94
6.. Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H, The spectrum of gastrointestinal metastases of breast carcinoma: I. Stomach: Gastrointest Endosc, 1992; 38; 130-35
7.. Mathew A, Rajagopal PS, Villgran V, Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast: Geburtshilfe Frauenheilkd, 2017; 77; 660-66
8.. Almubarak MM, Laé M, Cacheux W, Gastric metastasis of breast cancer: A single centre retrospective study: Dig Liver Dis, 2011; 43; 823-27
9.. Taal BG, Peterse H, Boot H, Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma: Cancer, 2000; 89; 2214-21
10.. De Gruttola I, Adil MT, D’Souza L, Perforated gastric carcinomatosis following invasive lobular cancer of the breast: Clin Case Rep, 2019; 7; 999-1002
11.. Asch MJ, Wiedel PD, Habif DV, Gastrointestinal metastases from crcinoma of the breast. Autopsy study and 18 cases requiring operative intervention: Arch Surg, 1968; 96; 840-43
12.. Akcali Z, Sakalli H, Ozyilkan O, Prognostically favorable abdominal breast cancer metastases with stomach involvement: Onkologie, 2005; 28; 270-72
13.. Kudo T, Matsumoto T, Nakamura S, Solitary minute metastasis from breast cancer mimicking primary intramucosal gastric signet-cell cancer: Gastrointest Endosc, 2005; 62; 139-40
14.. Whitty LA, Crawford DL, Woodland JH, Metastatic breast cancer presenting as linitis plastica of the stomach: Gastric Cancer, 2005; 8; 193-97
15.. Jones GE, Strauss DC, Forshaw MJ, Breast cancer metastasis to the stomach may mimic primary gastric cancer: Report of two cases and review of literature: World J Surg Oncol, 2007; 5; 75
16.. Dumoulin FL, Sen Gupta R, Breast cancer metastasis to the stomach resembling small benign gastric polyps: Gastrointest Endosc, 2009; 69; 174-75
17.. Pectasides D, Psyrri A, Pliarchopoulou K, Gastric metastases originating from breast cancer: Report of 8 cases and review of the literature: Anticancer Res, 2009; 29; 4759-63
18.. Vennapusa B, Oman SA, Parasher G, Cerilli LA, C-kit-positive gastric metastasis of lobular carcinoma of the breast masquerading as gastrointestinal stromal tumor: Breast Cancer, 2010; 17; 303-5
19.. Yamamoto D, Yoshida H, Sumida K, Gastric tumor from metastasis of breast cancer: Anticancer Res, 2010; 30; 3705-8
20.. Koike K, Kitahara K, Higaki M, Clinicopathological features of gastric metastasis from breast cancer in three cases: Breast Cancer, 2014; 21; 629-34
21.. Abid A, Moffa C, Monga DK, Breast cancer metastasis to the GI tract may mimic primary gastric cancer: J Clin Oncol, 2013; 31; e106-7
22.. Loubna M, Mohamed EH, Tijani EH, [Gastrointestinal metastases of breast cancer: report of 2 cases]: Pan Afr Med J, 2013; 15; 74
23.. Hild C, Talha-Vautravers A, Hoefler P, [Metastatic breast cancer to the stomach: An uncommon evolution of breast carcinoma]: Gynecol Obstet Fertil, 2014; 42; 47-50
24.. Eren OO, Ozturk MA, Sonmez O, Gastric metastasis in a patient with lobular breast carcinoma 6 years after diagnosis: J Gastrointest Cancer, 2014; 45; 504-5
25.. Waseda Y, Hayashi T, Kaneko S, Gastric metastasis from breast cancer visualized by magnifying endoscopy with narrow-band imaging: Dig Endosc, 2015; 27; 713
26.. Buka D, Dvořák J, Richter I, Gastric and colorectal metastases of lobular breast carcinoma: A case report: Acta Medica (Hradec Kralove), 2016; 59; 18-21
27.. El-Hage A, Ruel C, Afif W, Metastatic pattern of invasive lobular carcinoma of the breast-Emphasis on gastric metastases: J Surg Oncol, 2016; 114; 543-47
28.. Wong CS, Gumber A, Kiruparan P, Blackmore A, Gastric perforation secondary to metastasis from breast cancer: BMJ Case Rep, 2016; 2016; bcr2016214865
29.. Rodrigues MVR, Tercioti-Junior V, Lopes LR, Breast cancer metastasis in the stomach: When the gastrectomy is indicated?: Arq Bras Cir Dig, 2016; 29; 86-89
30.. Yim K, Ro SM, Lee J, Breast cancer metastasizing to the stomach mimicking primary gastric cancer: A case report: World J Gastroenterol, 2017; 23; 2251-57
31.. Barranco R, Orcioni GF, Ventura F, A fatal gastric perforation secondary to ulcerated metastasis in undiagnosed breast cancer: Pathological aspects and review of literature: Malays J Pathol, 2017; 39; 181-87
32.. Gurzu S, Banias L, Bara T, The epithelial-mesenchymal transition pathway in two cases with gastric metastasis originating from breast carcinoma, one with a metachronous primary gastric cancer: Recent Pat Anticancer Drug Discov, 2018; 13; 118-24
33.. Woo J, Lee J-H, Lee KE, Gastric metastasis as the first presentation one year before diagnosis of primary breast cancer: Am J Case Rep, 2018; 19; 354-59
34.. Rakha EA, Teoh TK, Lee AHS, Further evidence that E-cadherin is not a tumour suppressor gene in invasive ductal carcinoma of the breast: An immunohistochemical study: Histopathology, 2013; 62; 695-701
35.. Ferlicot S, Vincent-Salomon A, Médioni J, Wide metastatic spreading in infiltrating lobular carcinoma of the breast: Eur J Cancer, 2004; 40; 336-41
36.. Fernandes GS, Corrêa TS, Carvalho EPB, Gastric and endobronchial metastases in a case of lobular breast cancer: Case Rep Oncol, 2013; 6; 555-60
37.. Birla R, Dinu D, Iosif C, Constantinoiu S, Gastric metastasis of invasive lobular breast carcinoma, a current diagnostic and treatment challenge – a review: Chirurgia (Bucur), 2019; 114; 571-78
38.. Takeda Y, Tsuta K, Shibuki Y, Analysis of expression patterns of breast cancer-specific markers (mammaglobin and gross cystic disease fluid protein 15) in lung and pleural tumors: Arch Pathol Lab Med, 2008; 132; 239-43
39.. Meng X, Song S, Jiang Z, Receptor conversion in metastatic breast cancer: A prognosticator of survival: Oncotarget, 2016; 7; 71887-903
40.. Abrahao-Machado LF, Scapulatempo-Neto C, HER2 testing in gastric cancer: An update: World J Gastroenterol, 2016; 22; 4619-25
41.. Luo H-T, Liang C-X, Luo R-C, Gu W-G, Identification of relevant prognostic values of cytokeratin 20 and cytokeratin 7 expressions in lung cancer: Biosci Rep, 2017; 37; BSR20171086
42.. Ni Y-B, Tsang JYS, Shao M-M, GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer: Breast Cancer Res Treat, 2018; 169; 25-32
43.. Clarke M, Collins R, Darby S, Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials: Lancet, 2005; 366; 2087-106
44.. , Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials: Lancet, 2005; 365; 1687-717
45.. Hussain T, Elahi B, McManus P, Gastric obstruction secondary to metastatic breast cancer: A case report and literature review: J Med Case Rep, 2012; 6; 232
46.. Kim DH, Son S-M, Choi YJ, Gastric metastasis from invasive lobular breast cancer, mimicking primary gastric cancer: A case report: Medicine (Baltimore), 2018; 97; e0258
47.. Ogawa M, Namikawa T, Oki T, Gastric outlet obstruction caused by metastatic tumor of the stomach originating from primary breast cancer: A case report: Mol Clin Oncol, 2018; 9; 523-26
48.. Cardoso F, Costa A, Norton L, 1st International consensus guidelines for advanced breast cancer (ABC 1): Breast Edinb Scotl, 2012; 21; 242-52
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250