08 June 2021: Articles 
Endoscopic Ultrasound-Guided Transrectal Drainage of Perirectal Abscess in a Patient with Crohn Disease
Unusual setting of medical care
Ayesha Khalid1ABCDEF*, Mir Fahad Faisal1ACDEDOI: 10.12659/AJCR.930698
Am J Case Rep 2021; 22:e930698
Abstract
BACKGROUND: Crohn disease (CD) is an idiopathic chronic inflammatory disease that can present in the perianal area as perianal CD (pCD), which can present as fistulizing or non-fistulizing. Perirectal abscesses are common complications that are strongly associated with fistula formation. Draining an abscess and not treating the associated fistula leads to a high risk of disease recurrence. An extensive workup is needed to determine the nature and extent of disease and guide the appropriate treatment strategy. Endoscopic ultrasound (EUS) is an important modality for diagnosing CD-associated perianal or perirectal abscesses. It also has been used for treatment as an alternative to conventional surgical and percutaneous drainage techniques because it is minimally invasive and outcomes with it are good. The present report documents the case of a man with a history of CD who was diagnosed with a perirectal abscess that was managed with EUS-guided transrectal drainage.
CASE REPORT: A 58-year-old man with a history of CD presented with a 2-week history of chills, body aches, fatigue, and myalgia and a 1-week history of severe perirectal pain with worsening swelling. After a detailed history-taking, physical examination, and diagnostic workup, he was diagnosed with a CD-associated perirectal abscess. The patient and the attending physician decided to proceed with EUS-guided transrectal drainage.
CONCLUSIONS: Our case provides data regarding use of EUS for treatment of a CD- associated perirectal abscess.
Keywords: Abscess, Crohn Disease, Drainage, Fistula
Background
Crohn disease (CD) is an idiopathic chronic inflammatory disease characterized by segmental transmural inflammation that can involve any part of the gastrointestinal tract. The disease is often complicated by perianal CD (pCD), which is broadly classified into fistulizing (perianal and perirectal fistula/abscess) and non-fistulizing manifestations (skin tags, fissures, ulcers, strictures, hemorrhoids, and anal cancer) [1].
Pelvic and abdominal abscesses arise spontaneously in approximately 10% to 30% of patients with CD during the course of their illness [2,3]. Anorectal abscesses include perianal and perirectal abscesses. Perianal abscesses are superficial and can be diagnosed on physical examination as painful, tender, fluctuant swellings near the anal orifice. Perirectal abscesses, in contrast, are deep and form a tract that extends from the rectum into the pelvis. They can be found in various locations in the pelvis. Patients with them usually present with rectal pain that is worse with movement, straining, and fever.
Perianal and perirectal abscesses associated with CD are diagnosed based on imaging. They are strongly associated with fistula formation and studies have shown that abscess drainage while overlooking the associated fistula leads to a high risk of abscess recurrence [2,4]. Therefore, it is important to perform appropriate diagnostic tests to identify and classify a fistula. A detailed history-taking and physical examination followed by identification of active luminal inflammation via a small bowel series and colonoscopy are important because they form the basis of management decisions. Active inflammatory disease in the rectum and colon, such as proctitis, should not be managed with aggressive surgery because of the association with poor wound healing [5]. Studies have shown that combining an examination under anesthesia (EUA) with either endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI) of the pelvis produces a high yield in defining the extent and nature of pCD [6]. A multidisciplinary approach involving a gastroenterologist, colorectal surgeon, radiologist, pathologist, and nutritionist is necessary to provide optimal care and improve a patient’s quality of life. We present the case of a man with a history of CD who was diagnosed with a perianal and perirectal abscess for which he underwent EUS-guided transrectal rather than conventional surgical drainage.
Case Report
A 58-year-old man presented to the Emergency Department (ED) with a 2-week history of intermittent fever with chills, body aches, fatigue, and myalgia and a 1-week history of severe perirectal pain with worsening swelling. He reported that his pain was more severe during bowel movements and when he sat down, but had no abdominal pain, nausea, emesis, melena, hematochezia, or change in bowel habits. He noted that his symptoms were similar to the ones he had in 2010, when he developed a perirectal abscess that required surgical drainage. He had a history of CD, for which he had undergone multiple partial colectomies and a partial small bowel resection with ileocolonic anastomosis. The patient was being treated for his CD with infliximab infusions (100 mg i.v. once a month). He reported being allergic to metronidazole and having a 15-pack-year smoking history, but he had quit smoking 15 years ago. He had no family history of inflammatory bowel disease.
The patient was afebrile and tachycardiac (heart rate 114 bpm) at the time of presentation to the ED. On examination, a fluctuant swelling was found in his perianal area with surrounding erythema and tenderness; however, no fistulous tract with purulent drainage was seen. A review of other systems was unremarkable.
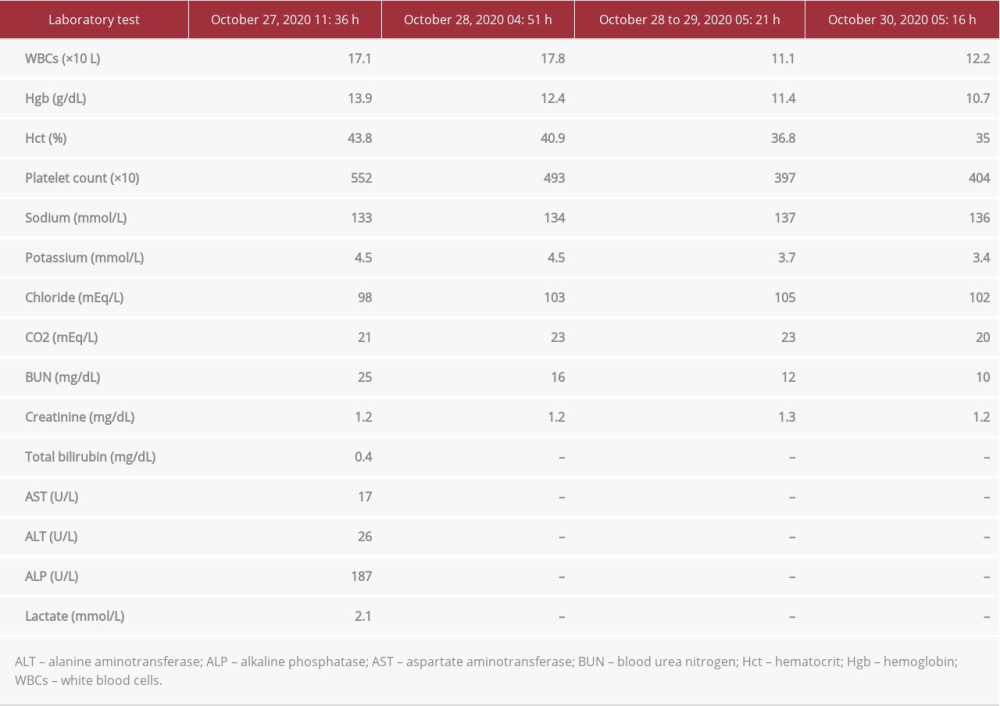
The initial laboratory analysis revealed leukocytosis with a white blood cell (WBC) count of 17.1×109/L and C-reactive protein (CRP) of 10.7 mg/dL (Table 1). Two blood samples were drawn and sent for culture. A contrast-enhanced computed tomography (CT) scan of the abdomen and pelvis revealed a 4.1×4.1×3.1 cm, rim-enhancing fluid collection in the right rectal wall near the anorectal junction (Figure 1). A left perianal fluid collection measuring 3.1×2.5 cm also was found, along with a small amount of cellulitis in the left gluteal cleft. Based on the patient’s history, physical examination, and imaging results, he was diagnosed with perianal sepsis in association with perianal and perirectal abscesses. He was started on intravenous fluids and an infusion of piperacillin/tazobactam (4.5 g/100 mL) and referred to a colorectal surgeon for surgical debridement. However, given that he had undergone multiple partial colectomies, a partial small bowel resection, and previous surgical drainage of a perirectal abscess, he was not in favor of having an external drain in place for weeks following CT-guided drainage of his abscess. After a detailed discussion about alternative treatments, he was referred to a gastroenterologist for EUS-guided transrectal drainage.
A colonoscopy was performed, which showed an ileocolonic anastomosis in the region of the proximal transverse colon. A patchy distribution of superficial erythematous erosions was seen, consistent with skip lesions; they, in turn, were consistent with mild ileitis. Cold forceps biopsies obtained from this region showed active inflammation in that area. No inflammatory changes were observed in the rectum; however, a left-sided bulge protruding into the lumen was noted and evaluated under EUS (Figure 2). The EUS findings included a hypoechoic cavity with regular walls and homogeneous material, suggestive of an abscess. The cavity measured 5×4 cm with no solid component or vascular signal (Figure 3). A 19-gauge needle was used for fine-needle aspiration. Purulent aspirate was sent for Gram staining and culture. A 0.025-inch, 450-cm-long guidewire with a hydrophilic tip was introduced and coiled into the abscess cavity (Figure 4). A tapered-tip 4F endoscopic retrograde cholangiopancreatography (ERCP) cannula was passed over the guidewire to dilate the tract. Further dilation was achieved using a 6 mm×4 cm biliary dilation balloon. A 7F, 3-cm double pigtail plastic biliary stent was placed but it failed to deploy, and given the distal location of the abscess, the stent was seen protruding from the anus. A needle knife was then used to access the cystic cavity, which was dilated using a 6 mm×4 cm biliary dilation balloon, followed by successful placement of a 10F, 5-cm double pigtail biliary-type plastic stent. After contrast injection, 2 abscess cavities were seen. The larger cavity was communicating only with the smaller one and there was some extravasation of contrast from the smaller cavity along the paracolic gutter, possibly indicating leakage of contents into this space from the abscess (Figure 5). Because the cavities were connected, a pigtail stent would allow drainage of both of them.
On the first day after the procedure, the patient reported feeling much better, with slight pain in the perirectal region. His leukocyte count improved from 17.1×109 L to 12.2×109 L. A contract-enhanced follow-up CT of the abdomen and pelvis performed 2 days after the procedure showed that the perirectal abscess was smaller (Figure 6). The patient was discharged home but presented to the ED later that day with a high fever. Cultures grew methicillin-resistant
Discussion
CD encompasses a spectrum of perianal complications, ranging from skin tags to fissures, ulcers, fistulas, and abscesses. Patients who report perianal or rectal symptoms should undergo a careful rectal examination and pelvic imaging. Imaging modalities with a high diagnostic sensitivity and specificity include MRI and EUS [7]. EUA is considered the standard for diagnosing pCD with a fistula. It is an important diagnostic modality for pCD and also facilitates simultaneous drainage of a perianal and/or perirectal abscess, leading to immediate relief of symptoms in patients [7]. A contrast-enhanced CT of the abdomen and pelvis can guide drainage of deep and complex perianal abscesses, but it has a limited role in diagnosing fistulizing pCD because of poor resolution and risk of exposure to ionizing radiation. Conventional treatment of a CD-associated anorectal abscess involves urgent surgical incision and drainage with placement of a mushroom catheter or a seton suture, if a fistula is present. CT- or US-guided percutaneous drainage is considered standard treatment for pelvic and perirectal abscesses; however, patients experience significant pain during and after the procedure and discomfort from in-dwelling catheters [8].
Recently, advanced endoscopic techniques have gained popularity for detection and treatment of CD-related complications, including strictures, obstructions, and abscesses. EUS-guided drainage is an alternative approach to surgical and percutaneous drainage of perianal and perirectal abscesses associated with CD. The literature describes case reports and case series about EUS-guided transrectal drainage of diverticular and postoperative pelvic abscesses; however, data are extremely limited regarding application of this approach in patients with pCD. Our patient and his surgeon were in favor of a nonsurgical approach after the complicated course of his disease, which had resulted in several surgeries and debridement. The patient reported significant improvement in symptoms within 24 h of EUS-guided transrectal drainage. A CT scan performed 48 h after the procedure showed that the abscess cavity was smaller.
A retrospective analysis of 4 cases of perirectal abscess (3 a result of postsurgical complications; 1 radiation proctitis) treated via EUS-guided transrectal drainage with the use of pigtail stents showed remarkable outcomes [8]. All 4 patients experienced immediate relief of symptoms with complete resolution of the abscess on CT performed 7 to 14 days later. The stents remained in place without migration and were removed via sigmoidoscopy during an outpatient visit. Median follow-up at 22 weeks revealed no recurrences.
In a 2-center case series, 37 patients underwent EUS-guided drainage of perirectal and perisigmoid abscesses (31 were post-surgical while 6 were due to medical conditions including CD) with use of plastic or lumen-apposing metal stents (LAMS) [9]. All showed resolution of the abscess on follow-up CT at 4 weeks, with significant improvement in symptoms. Three patients had recurrences at 3 and 12 months and needed surgical drainage.
A case series of 8 patients with pelvic abscesses who underwent EUS-guided drainage was retrospectively analyzed [10]. The underlying etiology of the abscesses was diverticulitis in 4 patients, postsurgical complications in 2, CD in 1, and iatrogenic in 1 patient. The results showed complete resolution of symptoms in all 8 patients, with no recurrences of the abscesses during a follow-up period of 38 months. The patients became afebrile within 24 h of the procedure and follow-up CT scans showed complete disappearance of the abscesses. Endoscopic removal of a stent was performed in 2 patients, while the remaining 6 had spontaneous dislodgement of stents to the outside. In the patient with CD, ileocecal resection was performed 2 months later.
Meylemans et al performed a retrospective cohort study to assess the safety and effectiveness of EUS-guided transrectal drainage vs surgical drainage of pelvic abscesses from different causes [11]. The success rate (defined as no need for further treatment or intervention) in the EUS-guided drainage group was reported to be much higher than in the surgical drainage group (83% vs 48%, respectively). The duration of abscess drainage was longer in the EUS-guided drainage group (42 vs 13 days). The lengths of hospital stay and abscess resolution were similar in the 2 groups.
A retrospective analysis was conducted of EUS-guided transrectal drainage of pelvic fluid collections (PFC) using LAMS [12]. The study included 5 patients; 4 had postoperative PFC while 1 had an abscess that arose as a complication of acute diverticulitis. In 2 of the 5 cases, fecal diversion occurred; 1 had a concomitant abdominal abscess, which was drained percutaneously during the same period. A 100% clinical success rate was achieved and the LAMS was removed after a median of 14 days. No recurrences were observed during a follow-up period of 14 months. In comparison with plastic stents and drainage catheters, the use of LAMS for EUS-guided drainage of pelvic abscesses is more efficacious with better outcomes and minimal adverse events, such as dislodgement, migration, or stent contamination with fecal matter. Use of LAMS leads to more rapid abscess resolution compared to EUS-guided drainage without LAMS. Because our patient’s abscess was 2 to 3 cm from his anal orifice, it was felt that using a LAMS would cause him significant irritation and discomfort; therefore, the decision was made to use a plastic stent instead.
Conclusions
EUS-guided transrectal drainage has gained popularity in the last 2 decades. We have presented a case that illustrates a successful outcome with use of this procedure in a patient with pCD. Because very limited data are available on use of this minimally invasive technique for a CD-associated perirectal abscess, our experience contributes to a better understanding of this treatment and its related outcomes in patients with pCD.
Figures
References:
1.. Satsangi J, Silverberg MS, Vermeire S, Colombel JF, The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications: Gut, 2006; 55; 749-53
2.. Richards RJ, Management of abdominal and pelvic abscess in Crohn’s disease: World J Gastrointest Endosc, 2011; 3(11); 209-12
3.. Hamada T, Kosaka K, Sonde C, A case of abdominal abscess in Crohn’s disease: Successful endoscopic demonstration of an obscure enteric fistula by dye injection via a percutaneous drainage catheter: Case Rep Gastroenterol, 2009; 3; 138-46
4.. Rypens F, Dubois J, Garel L, Percutaneous drainage of abdominal abscesses in pediatric Crohn›s disease: AJR Am J Roentgenol, 2007; 188; 579-85
5.. Lewis RT, Bleier JI, Surgical treatment of anorectal Crohn disease: Clin Colon Rectal Surg, 2013; 26(2); 90-99
6.. Schwartz DA, Wiersema MJ, Dudiak KM, A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas: Gastroenterology, 2001; 121; 1064-72
7.. Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Management of complex perianal Crohn’s disease: Ann Gastroenterol, 2017; 30(1); 33-44
8.. Choi EK, Kim JH, Jeong SU, Endoscopic ultrasound-guided perirectal abscess drainage without drainage catheter: A case series: Clin Endosc, 2017; 50(3); 297-300
9.. Poincloux L, Caillol F, Allimant C, Long-term outcome of endoscopic ultrasound-guided pelvic abscess drainage: A two-center series: Endoscopy, 2017; 49(5); 484-90
10.. Hadithi M, Bruno MJ, Endoscopic ultrasound-guided drainage of pelvic abscess: A case series of 8 patients: World J Gastrointest Endosc, 2014; 6(8); 373-78
11.. Meylemans DVG, Oostenbrug LE, Endoscopic ultrasound guided versus surgical transrectal drainage of pelvic abscesses: Acta Chir Belg, 2018; 118(3); 181-87
12.. Lisotti A, Cominardi A, Bacchilega I, EUS-guided transrectal drainage of pelvic fluid collections using electrocautery-enhanced lumen-apposing metal stents: A case series: VideoGIE, 2020; 5(8); 380-85 29
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250