30 July 2021: Articles 
A 40-Year-Old Man with Sarcoidosis and Factor V Leiden Thrombophilia Presenting with Deep Vein Thrombosis and Pulmonary Thromboembolism
Rare coexistence of disease or pathology
Anna Goljan-Geremek1ABCDEF*, Elżbieta Puścińska2CD, Witold Tomkowski3DE, Paweł Śliwiński2DEDOI: 10.12659/AJCR.932286
Am J Case Rep 2021; 22:e932286
Abstract
BACKGROUND: The association between sarcoidosis and pulmonary embolism (PE) has been described in the literature, but little is known about the origin of hypercoagulability and hypofibrinolysis in sarcoidosis. PE is a multifactorial disease that is rarely caused by a single risk factor, and might be expected in disabling sarcoidosis. No data are available, however, about sarcoidosis being a risk factor for venous thromboembolism in factor V Leiden thrombophilia.
CASE REPORT: We describe a case of a 40-year-old man with asymptomatic sarcoidosis. Diagnosis was based on abnormal chest radiology (enlargement of hilar and mediastinal lymph nodes), confirmed by histopathological examination (noncaseating granulomas involving the mediastinal lymph nodes). No therapy was proposed due to good exercise tolerance, normal pulmonary function test, and absence of extrapulmonary involvement. The patient was followed up for 5 years until he developed progressive exertional dyspnea and chest pain. Plasma D-dimers, serum NT-proBNP, and troponin were increased. A computed tomography angiogram confirmed PE. Factor V Leiden thrombophilia was diagnosed following a search for risk factors for thromboembolism. Spontaneous remission of the chest lymphadenopathy was observed on anticoagulation therapy. Different potential mechanisms that relate sarcoidosis to venous thromboembolism are discussed.
CONCLUSIONS: PE is a potentially fatal condition and may complicate sarcoidosis, a clinically insignificant condition. Sarcoidosis patients with new symptomatology and PE with a high concentration of plasma D-dimers merit extra consideration. In certain clinical situations, sarcoidosis may be considered as a risk factor for deep vein thrombosis/PE. The anti-inflammatory and anti-fibrotic properties of anticoagulation warrant further study.
Keywords: Embolism and Thrombosis, factor V Leiden, Sarcoidosis, Pulmonary, Pulmonary Embolism, case reports, Activated Protein C Resistance, Factor V, sarcoidosis, Thrombophilia, venous thromboembolism, Venous Thrombosis
Background
Venous thromboembolism (VTE) is a condition seen frequently in Western populations, with an incidence of 1000 persons per year [1]. Two or more risk factors can lead an individual to develop this potentially harmful condition [2]. Although the association between sarcoidosis and pulmonary embolism (PE) have been reported recently, little is known about the origin of hypercoagulability and hypofibrinolysis in sarcoidosis.
Macrophages and activated leukocytes, which play key roles in the inflammatory process of sarcoidosis, are potent stimulants of thrombin and fibrin formation [3]. PE, a multifactorial disease, is rarely caused by a single risk factor and might not be uncommon in advanced sarcoidosis associated with known risk factors for VTE. No data are available about the incidence of VTE in recently diagnosed, active sarcoidosis, and data about the clinical characteristics of sarcoidosis complicated by VTE are limited [4,5]. Factor V Leiden thrombophilia (FVLT) is a known risk factor for VTE. The aim of our case report is to present a clinical picture of active sarcoidosis, which might be considered as an additional risk factor for developing VTE.
Case Report
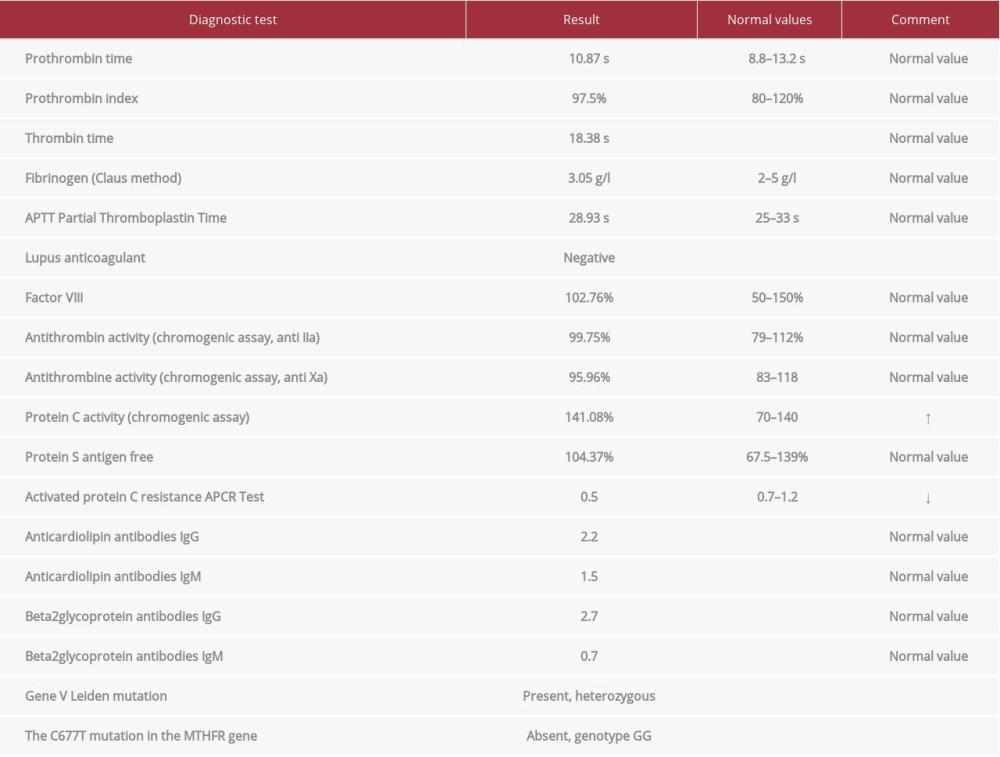
A 40-year-old man was admitted to the cardiology department because of exertional dyspnea of sudden onset and chest pain; heart rate was 125 beats/min, systemic blood pressure was 120/75 mmHg, and arterial oxygen saturation (SpO2) when breathing room air was 90%. Five years prior to admission, he was diagnosed with stage I sarcoidosis according to Scadding classification (Figure 1). Sarcoidosis was confirmed by lymph node biopsy via cervical mediastinoscopy, which showed multiple, well-formed granulomas consisting of predominantly epithelioid cells, giant cells with a peripheral rim of inflammatory cells, and fibrosis (Figure 2). To rule out tuberculosis, a Ziehl-Neelsen-negative necrotizing granulomatous reaction was documented in lymph node tissue, and confirmed by culture and PCR. Microbiological examinations performed on bronchoalveolar lavage (smear, culture, and PCR) were negative for acid-fast bacilli (mycobacteria) and fungi. The patient was asymptomatic, with a stable radiological picture (hilar and mediastinal lymphadenopathy), and remained without treatment. Upon admission to the cardiology department, abnormal laboratory test results were obtained, as follows: plasma D-dimer level (DD): 6250 mcg/L (N: 68–494); serum NT-proBNP 2319 pg/mL (N: <93pg/mL); troponin 0.619 ng/mL (N: 0.034 ng/mL). Abnormal findings in transthoracic echo-cardiography were: right ventricular systolic pressure (RVSP), 50 mmHg; pulmonary truncus, 24 mm; shortened acceleration time (AcT), 45 ms; inferior vena cava (16 mm) with low respiratory variability. A computed tomography (CT) angiogram revealed massive bilateral pulmonary artery emboli in almost all segmental arteries (Figure 3). Doppler ultrasound showed deep vein thrombosis (DVT) in the right-side femoral distal, proximal popliteal, and saphenous veins. Low-molecular-weight heparin treatment was started, and this resulted in rapid clinical improvement and normalization of laboratory markers of cardiac dysfunction and DD. The clinical picture of sarcoidosis when PE was discovered consisted of: abnormal conventional X-ray (CXR), normal PFT, and no extrapulmonary involvement. Screening for surgery, trauma, immobilization previous to DVT, and malignancy, all of which are recognized procoagulant risk factors, was negative. Repeated testing for antinuclear antibodies (ANA), lupus anticoagulant (LAC), rheumatoid factor, anticardiolipin antibodies (ACLA) (IgG, IgA, IgM), and beta2-glucoprotein antibodies (IgG, IgM) was negative. Antithrombin, protein S, and protein C were within normal limits. Molecular diagnosis for factor V Leiden, the G20210A mutation in the prothrombin gene, and the C677T mutation in the methylenetetrahydrofolate reductase (MTHFR) gene was performed as a routine search for thrombophilia performed in the Institute of Hematology and Blood transfusion in Warsaw (Table 1).
Molecular assessment of factor V Leiden was performed on DNA extracted from blood samples by the salting out method of Miller et al. Polymerase chain reaction (PCR) of a fragment of exon 10 of the factor V Leiden gene was carried out, followed by digestion of the PCR product with
Discussion
A UK study showed significantly increased risk of VTE in sarcoidosis patients, compared with a reference cohort [4]. According to data from the National Center for Health Statistics, among U.S. patients, sarcoidosis cases were associated with DVT, without any association related to age, race, or gender [9]. The mechanisms underlying the association between sarcoidosis and VTE are unknown. Thrombus formation may affect a diversity of organs in sarcoidosis. Cases reported in the literature show that VTE occurs in proximity to organs with active inflammatory processes: mural thrombus in myocardial sarcoidosis [10], vein thrombosis in neurosarcoidosis [11,12], thoracic vein thrombosis in mediastinal disease [13], and portal vein thrombosis in hepatic sarcoidosis [14]. Granulomatous inflammation in neighboring tissues may induce excessive coagulation and lead to local thromboembolism. Swigris et al found that sarcoidosis decedents with PE were less likely than those without PE to have certain other conditions that might have predisposed them to death (myocardial infarction, heart failure, cardiac dysrhythmia, sudden cardiac death, or pneumonia) [9]. The authors concluded that active inflammation with no others risk factors for DVT may be responsible for hypercoagulation in sarcoidosis cases. Recent studies have demonstrated a probable association between VTE and several markers of inflammation [15,16]. Tumor necrosis factor (TNF)-α, interleukin 6 (IL-6), and interleukin 8 (IL-8) levels have been shown to be risk determinants for venous thrombosis. TNF-α is a crucial cytokine in granuloma formation and an indicator of inflammation activity in sarcoidosis [3,17,18]. There is a significant correlation between inflammatory processes and vascular function impairment in sarcoidosis [19]. Those observations may partially explain the possible relation between active sarcoidosis and high risk for VTE, as both conditions share the same set of cytokines. Elevated local or generalized TNF-α concentration might initiate thromboembolic events. Interleukin 10 (IL-10), an anti-inflammatory cytokine, was found to be protective against venous thrombosis [15]. Increased local secretion of IL-10 may induce a downmodulating mechanism leading to resolution of sarcoid alveolitis [17]. A favorable course of sarcoidosis driven by anti-inflammatory and anti-thrombotic cytokines like IL-10 might be associated with a low risk for VTE. There are no data about the clinical aspects of sarcoidosis and its inflammatory status in patients who were diagnosed with PE and sarcoidosis [4].
Circulating DD are frequently positive in patients with sarcoidosis, and are associated with disease activity [20]. Associations have been shown between DD and the radiographic stage of sarcoidosis, diffusing capacity of the lung for carbon monoxide (DLCO), and dyspnea [20]. At initial evaluation, our patient showed a high level of DD despite the mild manifestation of sarcoidosis. High DD level reflects hypercoagulation status rather than being a marker of the activity and unfavorable prognosis of sarcoidosis. In stable sarcoidosis, increased DD associated with new symptoms deserves further investigation.
The factors that increase the risk for VTE in sarcoidosis are the subject of speculation. They probably depend on the intensity of inflammation, organ involvement, disability, and comorbidities [4,9]. In our patient, the risk for VTE was driven by FVLT. According to the literature, in 50% of individuals with FVLT, thromboembolism will develop when other predisposing components are present [21]. We have found one case of sarcoidosis and FVLT with cerebral DVT described in the literature [12]. The authors of that report concluded that since most individuals heterozygous for the factor V Leiden gene do not develop thromboembolism, the clinical manifestations of DVT/PE will show up only when an inherited predisposition (heterozygous FVLT) is comorbid with an acquired thrombogenic stimulus (sarcoidosis). In this context, sarcoidosis can be considered as a risk factor for DVT/PE. Resolution of pulmonary changes in a patient on anticoagulation therapy for DVT has been reported [22]. The favorable outcome of sarcoidosis in our patient is consistent with this observation. Heparins possess anti-inflammatory properties that inhibit vein wall neutrophils and total inflammatory cells, while decreasing vein wall permeability. However, it has not been proven whether a cytokine-mediated anti-inflammatory effect might play a special role in sarcoidosis [23].
Conclusions
PE may be responsible for fatal outcomes linked to clinically irrelevant sarcoidosis. Further investigations of VTE complicating sarcoidosis should be continued, as the co-existence of both conditions is more common than expected. In certain clinical situations, as described above, sarcoidosis may be considered as a risk factor for DVT/PE. The anti-inflammatory properties of anticoagulants warrant further study.
Figures
References:
1.. Heit JA, The epidemiology of venous thromboembolism in the community: Arterioscler Thromb Vasc Biol, 2008; 28; 370-72
2.. Rosendaal FR, Venous thrombosis: A multicausal disease: Lancet, 1999; 353; 1167-73
3.. Hasday JD, Bachwich PR, Lynch JP, Sitrin RG, Procoagulant and plasminogen activator activities of bronchoalveolar fluid in patients with pulmonary sarcoidosis: Exp Lung Res, 1988; 14; 261-78
4.. Crawshaw AP, Wotton CJ, Yeates DG, Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study: Thorax, 2011; 66; 447-48
5.. Goljan Geremek A, Tomkowski W, Geremek M, Sarcoidosis as a risk factor for venous thromboembolism: Sarcoidosis Vasc Diffuse Lung Dis, 2017; 34(2); 170-78
6.. Bertina RM, Koeleman BP, Koster T, Mutation in blood coagulation factor V associated with resistance to activated protein C: Nature, 1994; 369(6475); 64-67
7.. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM, A common genetic variation in the 3’-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis: Blood, 1996; 88(10); 3698-703
8.. Frosst P, Blom HJ, Milos R, A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase: Nat Genet, 1995; 10(1); 111-13
9.. Swigris JJ, Olson AL, Huie TJ, Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007: Chest, 2011; 140; 1261-66
10.. Wynne JW, Ryerson GG, Dalovisio J, Myocardial sarcoidosis complicated by mural thrombosis: Thorax, 1979; 34; 127-29
11.. Akova YA, Kansu T, Duman S, Pseudotumor cerebri secondary to dural sinus thrombosis in neurosarcoidosis: J Clin Neuroophthalmol, 1993; 13; 188-89
12.. Selvi A, Diakou M, Giannopoulos S, Cerebral venous thrombosis in a patient with sarcoidosis: Intern Med, 2009; 48; 723-25
13.. Marc K, Bourkadi JE, Benamor J, Iraqi G, Thoracic venous thrombosis in the course of sarcoidosis: Rev Mal Respir, 2008; 25; 105-6
14.. Moreno-Merlo F, Wanless IR, Shimamatsu K, The role of granulomatous phlebitis and thrombosis in the pathogenesis of cirrhosis and portal hypertension in sarcoidosis: Hepatology, 1997; 26; 554-60
15.. van Aken BE, Den HM, Bos GM, van Deventer SJ, Reitsma PH, Recurrent venous thrombosis and markers of inflammation: Thromb Haemost, 2000; 83; 536-39
16.. Reitsma PH, Rosendaal FR, Activation of innate immunity in patients with venous thrombosis: The Leiden Thrombophilia Study: J Thromb Haemost, 2004; 2; 619-22
17.. Bingisser R, Speich R, Zollinger A, Interleukin-10 secretion by alveolar macrophages and monocytes in sarcoidosis: Respiration, 2000; 67; 280-86
18.. Baydur A, Alavy B, Nawathe A, Fatigue and plasma cytokine concentrations at rest and during exercise in patients with sarcoidosis: Clin Respir J, 2011; 5; 156-64
19.. Siasos G, Tousoulis D, Gialafos E, Association of sarcoidosis with endothelial function, arterial wall properties, and biomarkers of inflammation: Am J Hypertens, 2011; 24; 647-53
20.. Shorr AF, Hnatiuk OW, Circulating D dimer in patients with sarcoidosis: Chest, 2000; 117; 1012-16
21.. Heit JA, Sobell JL, Li H, Sommer SS, The incidence of venous thromboembolism among Factor V Leiden carriers: A community-based cohort study: J Thromb Haemost, 2005; 3; 305-11
22.. Hedfors E, Anticoagulant treatment in sarcoidosis: Acta Med Scand, 1977; 202; 237-40
23.. Downing LJ, Strieter RM, Kadell AM, Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis: J Vasc Surg, 1998; 28; 848-54
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250