25 August 2021: Articles 
Transition From Distinct Types of Mutation-Harboring Multifocal Lung Adenocarcinoma to Rhabdoid Tumor: A Longitudinal Follow-Up
Challenging differential diagnosis, Rare disease
Kensuke Setoguchi1ABCDEF, Shigehisa Yanagi1ABCDEFG*, Toshihiro GiDOI: 10.12659/AJCR.932452
Am J Case Rep 2021; 22:e932452
Abstract
BACKGROUND: Rhabdoid tumor (RT) of the lung is a rare and aggressive malignancy. The origin of and the mutation responsible for RT are entirely unknown. The distinction between RT associated with subtypes of lung cancer and SMARCA4-deficient thoracic sarcomas is also unknown.
CASE REPORT: Three pulmonary subsolid nodules in the right S6, left S6, and left S8 were identified in a 78-year-old Japanese woman. At 3 and 9 months later, a chest CT showed unchanged sizes, but at 15 months the development of a 37-mm mass in the right S6 was observed. The patient’s systemic condition deteriorated rapidly, and she died 1 month later. An autopsy revealed that the mass consisted of 90% RT and 10% lung adenocarcinoma. There were another 2 adenocarcinoma lesions in the left lung. KRAS mutation analyses revealed the same KRAS mutation (G12D) in the adenocarcinoma and RT components in the identical mass and metastatic RT, indicating that all of these components had the same clonality. A different KRAS mutation in each of the 3 adenocarcinoma lesions was detected (right S6: G12D, left S6: A59G, left S8: G12C), indicating that the multiple adenocarcinoma lesions were truly multifocal lung adenocarcinoma. The adenocarcinoma and RT components retained SMARCA4 expression.
CONCLUSIONS: This is the first evidence of RT originating from multifocal lung adenocarcinoma. KRAS mutation is thought to be responsible for the RT’s emergence via the epithelial-mesenchymal transition. Patients with multiple subsolid nodules should be followed closely; aggressive surgical intervention should be considered given concerns about the evolution of this aggressive malignancy.
Keywords: SMARCA2 Protein, Human, Rhabdoid Tumor, KRAS Protein, Human, Adenocarcinoma of Lung, DNA Helicases, Follow-Up Studies, Mutation, Nuclear Proteins, Proto-Oncogene Proteins p21(ras), Transcription Factors
Background
Rhabdoid tumor (RT), a highly aggressive neoplasm, was first described in 1978 as a childhood-onset distinctive renal tumor [1]. Several cases of adult-onset RTs have been reported in the kidneys as well as extrarenal sites including the lungs [2,3]. In the 2004 World Health Organization (WHO) classification, large-cell carcinoma with rhabdoid phenotype (LCC-RP) was grouped as a variant type of large-cell carcinoma [4]. LCC-RP is defined as having malignant tumor cells comprised of ≥10% rhabdoid cells, which are characterized by abundant acidophilic cytoplasm, large nuclei, and conspicuous eosinophilic cytoplasmic globules [5]. LCC-RP is extremely rare, and it is an aggressive malignancy with a poor prognosis [5,6].
In the 2015 WHO classification, the rhabdoid phenotype was regarded as a cytologic feature rather than a specific histologic group, as it colocalizes with various histologic subtypes [7,8]. More recently, SMARCA4 (SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 4)-deficient thoracic sarcomas (SMARCA4-DTSs) were proposed as a distinctive disease entity with rhabdoid morphology and highly aggressive malignancy [9]. To date, direct evidence of the cell lineage in which RTs arise from a specific subtype of lung cancer has not been obtained. The driver oncogene(s) responsible for the occurrence of RT in lung cancer are also not fully understood. Moreover, the distinction between RT associated with subtypes of lung cancer and SMARCA4-DTSs remains to be determined.
Here, we report a patient with RT arising from multifocal lung adenocarcinoma. The results of our longitudinal chest computed tomography (CT) and
Case Report
A 78-year-old Japanese woman presented with a 1-month history of dyspnea on effort. She had never smoked and had no history of alcohol use or dust exposure. She had a 32-year history of systemic lupus erythematosus, and she had been continuously treated with oral prednisolone (4 mg/day) and mizoribine (50 mg/day). She had undergone radical surgery for ascending colon cancer 4 years before her presentation. The chest CT at presentation demonstrated 3 subsolid nodules in her lung fields: an 11-mm subsolid nodule in the superior (S6) segment of the right lower lobe, a 9-mm subsolid nodule in the superior (S6) segment of the left lower lobe, and an 8-mm subsolid nodule in the anteromedial (S8) segment of the left lower lobe (Figure 1).
A transbronchial biopsy was performed for the lesion in the right S6 segment, but no definite diagnosis was made. The patient was then followed-up by chest CT assessments, and the chest CT examinations conducted 3 and 9 months later showed that the nodules had remained unchanged in size. However, a chest CT at 15 months demonstrated the development of a 37-mm mass lesion in the right S6 segment. A CT-guided needle biopsy was performed for the mass lesion at 16 months. The pathology assessment of the needle-biopsied specimen revealed that the mass lesion was composed of carcinoma cells with rhabdoid features, characterized by large cells with eccentrically located nuclei, prominent nucleoli, abundant eosinophilic cytoplasm, and large intracytoplasmic inclusions. The tumor cells were negative for
Immunohistochemistry results showed that the programmed death-1 ligand-1 tumor proportion score of the tissue sample was 50%. A chest CT examination revealed the rapid growth of the mass lesion (from 37 mm to 60 mm within 1 month) (Figure 1). The subsolid nodules in the left S6 and S8 segments were unchanged in size throughout the clinical course. At 17 months, the patient was hospitalized with fever, wet cough, bloody sputum, and appetite loss. Her systemic condition deteriorated rapidly, and her Eastern Cooperative Oncology Group performance status fell to 4. We therefore decided to manage her treatment as best supportive care. She died 1 month after being hospitalized.
The autopsy revealed that there was a 65-mm mass lesion in the right S6 segment with extensive hemorrhage and necrosis (Figure 2A). The mass consisted of 90% solid tumor with rhabdoid cells and 10% non-mucinous adenocarcinoma lesion with focal intracytoplasmic mucin (Figure 2B–2D). A continuum of changes from adenocarcinoma to the solid area with rhabdoid cells was observed, suggesting the process of epithelial-mesenchymal transition (EMT) (Figure 2E). The adeno-carcinoma lesion was positive for cytokeratin (CK) AE1/AE3 (a pan-cytokeratin marker), CAM5.2 (a pan-cytokeratin marker), CK7, and CK20 (Figure 3A, 3C, 3F, 3G). Mucin 5AC (MUC5AC) immunopositivity was detected in a small part of the adeno-carcinoma lesion (Figure 3H). The immunoreactivity for hepatocyte nuclear factor 4α (HNF4α) was scant (Figure 3I). Rhabdoid cells were positive for AE1/AE3, CAM5.2, and vimentin but negative for CK7, CK20, MUC5AC, and HNF4α (Figure 3B, 3D, 3E). Multiple metastatic foci of RT were observed in the pancreas, lungs, heart, gall bladder, and soft palate. There were another 2 adenocarcinoma lesions in the S6 and S8 segments of the left lower lobe (Figure 4A–4E). There was no evidence of the recurrence of the preexisting colon cancer.
To investigate whether the adenocarcinomas and RT had the same origin, we analyzed
The
We then investigated the involvement of
Discussion
Rhabdoid tumor of the lung is an extremely rare type of lung cancer characterized by a highly aggressive malignancy property. The origin of RT and its responsible driver oncogene remain unclear. This is the first case report identifying an RT that had originated from a multifocal adenocarcinoma in the lung. The longitudinal CT findings and the results of our
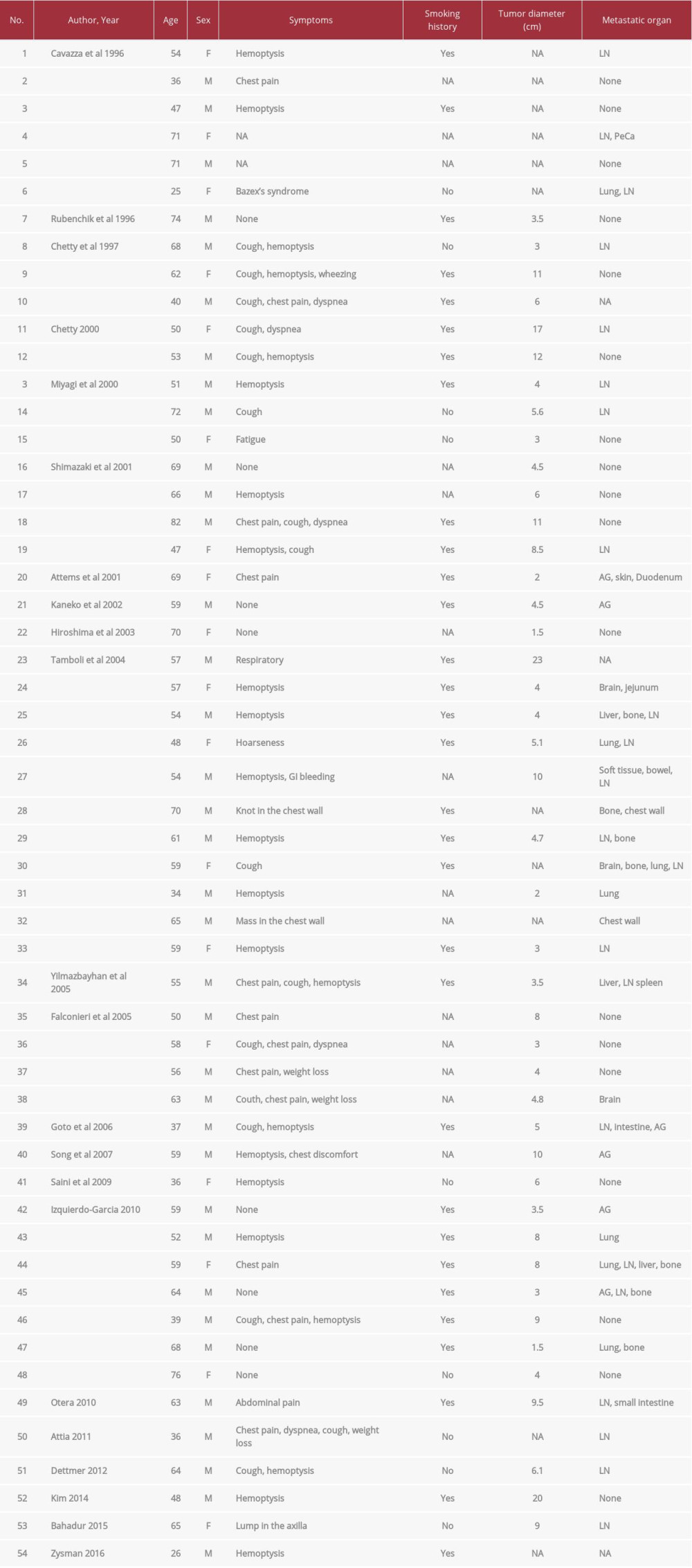
Several case studies have reported RTs in the lung that co-localized with various subgroups of differentiated lung cancer. Based on our literature search, as of March 20, 2021, 54 cases of RT in the lung have been described in 22 published articles (Table 1) [5,6,15–34]. Among these cases, 50 case reports noted the associated tumor types: adenocarcinoma (30%, 15 of the 50 cases), large-cell carcinoma (28%, 14 cases), poorly or undifferentiated tumor (10%, 5 cases), sarcomatoid carcinoma (8%, 4 cases), large-cell neuroendocrine carcinoma (6%, 3 cases), squamous cell carcinoma (4%, 2 cases), small cell carcinoma (4%, 2 cases), invasive mucinous adenocarcinoma (IMA, 2%, one case), and more. This is the first case report to describe a patient with RT associated with multifocal lung adenocarcinoma.
Since some of the adenocarcinoma cells contained intracytoplasmic mucin in our patient’s case, we initially considered IMA as a differential diagnosis of non-mucinous lung adenocarcinoma. However, several of the findings were different from the essential and desirable diagnostic criteria of IMA described in the most recent published WHO classification of thoracic tumors [35]. First, almost none of tumor cells in our patient’s case had abundant apical columnar cells with small basally oriented nuclei. Second, the immunopositivity of MUC5AC and HNF4α, which are markers of IMA, was scant in the present case. A recent study with a comprehensive genomic analysis demonstrated the clonal relationship of spatially separate IMA lesions: among 24 patients with 2 separate IMAs, tumors from all but 1 patient shared the same driver mutations [36]. In contrast, in the present study, each adenocarcinoma lesion possessed a different type of KRAS mutation. We thus concluded that all 3 of these adenocarcinomas were not IMAs, but rather were non-mucinous adenocarcinomas with intracytoplasmic mucin.
In our patient’s case, 4 findings indicated that the multifocal adenocarcinoma underwent a transition to RT. First, the histology showed the presence of the continuum of changes from adenocarcinoma to the solid area with rhabdoid cells, suggesting the EMT process. Second, the immunohistochemical study exhibited continuous changes of epithelial- and mesenchymal-marker expression between the adenocarcinoma and RT areas. Third, the
The EMT plays pivotal roles in cancer biology including tumor growth, invasion, dissemination, and metastasis [37]. Since EMT-suggestive findings were observed in the RT specimen in the lung in the present and previous cases [26,31], the EMT might be key to the dedifferentiation of parental cancer cells to rhabdoid cells in the lung. Regarding the mutation responsible for RT evolution, Dettmer et al showed the existence of the same
In 2015, Le Loarer and colleagues demonstrated that
Another important feature of our patient’s case was the rapid growth of the mass during the CT follow-up for the management of the multiple subsolid nodules of the lung. The 2017 Fleischner Society Guidelines recommend that incidental pulmonary nodules be managed as follows: in patients with multiple subsolid lesions ≥6 mm, a short-term follow-up CT at 3–6 months should be considered [45]. Our patient’s case emphasizes the necessity of considering aggressive surgical intervention (eg, multiple limited resections) in patients with multiple subsolid lesions in order to terminate the evolution of the RT.
Conclusions
We present the first case of a rhabdoid tumor arising from multifocal lung adenocarcinoma.
Figures
References:
1.. Beckwith JB, Palmer NF, Histopathology and prognosis of Wilms tumors: Results from the First National Wilms’ Tumor Study: Cancer, 1978; 41; 1937-48
2.. Parham DM, Weeks DA, Beckwith JB, The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy: Am J Sur Pathol, 1994; 18; 1010-29
3.. Wick MR, Ritter JH, Dehner LP, Malignant rhabdoid tumors: A clinicopatho-logic review and conceptual discussion: Semin Diagn Pathol, 1995; 12; 233-48
4.. Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC: Pathology and genetics: Tumours of the lung, pleura, thymus and heart,; 2004, Lyon, IARC
5.. Cavazza A, Colby TV, Tsokos M, Lung tumors with a rhabdoid pheno-type: Am J Clin Pathol, 1996; 105; 182-88
6.. Shimazaki H, Aida S, Sato M, Lung carcinoma with rhabdoid cells: A clinicopathological study and survival analysis of 14 cases: Histopathology, 2001; 38; 425-34
7.. Travis WD, Brambilla E, Burke AP: WHO Classification of tumours of the lung, pleura, thymus and heart, 2015, Lyon, International Agency for Research on Cancer
8.. Travis WD, Brambilla E, Nicholson AG, The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification: J Thorac Oncol, 2015; 10; 1243-60
9.. Le Loarer F, Watson S, Pierron G, SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas: Nat Genet, 2015; 47; 1200-5
10.. Nordgård O, Oltedal S, Janssen EA, Comparison of a PNA clamp PCR and an ARMS/Scorpion PCR assay for the detection of K-ras mutations: Diagn Mol Pathol, 2012; 21; 9-13
11.. Perret R, Chalabreysse L, Watson S, SMARCA4-deficient thoracic sarcomas: Clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses: Am J Surg Pathol, 2019; 43; 455-6
12.. Rekhtman N, Montecalvo J, Chang JC, SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas: J Thorac Oncol, 2020; 15; 231-47
13.. Sauter JL, Graham RP, Larsen BT, SMARCA4-deficient thoracic sarcoma: A distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior: Mod Pathol, 2017; 30; 1422-32
14.. Yoshida A, Kobayashi E, Kubo T, Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities: Mod Pathol, 2017; 30; 797-809
15.. Rubenchik I, , Dardick I, Auger M. Cytopathology and ultrastructure of primary rhabdoid tumor of lung: Ultrastruct Pathol, 1996; 20; 355-60
16.. Chetty R, Bhana B, Batitang S, Govender D, Lung carcinomas composed of rhabdoid cells: Eur J Surg Oncol, 1997; 23; 432-34
17.. Chetty R, Combined large cell neuroendocrine, small cell and squamous carcinomas of the lung with rhabdoid cells: Pathology, 2000; 32; 209-12
18.. Miyagi J, Tsuhako K, Kinjo T, Rhabdoid tumour of the lung is a de-differentiated phenotype of pulmonary adenocarcinoma: Histopatology, 2000; 37; 37-44
19.. Attems JH, Lintner F, Pseudomesotheliomatous adenocarcinoma of the lung with rhabdoid features: Pathol Res Pract, 2001; 197; 841-46
20.. Kaneko T, Honda T, Fukushima M, Large cell carcinoma of the lung with a rhabdoid phenotype: Pathol Int, 2002; 52; 643-47
21.. Hiroshima K, Shibuya K, Shimamura F, Pulmonary large cell carcinoma with rhabdoid phenotype: Ultrstruct Pathol, 2003; 27; 55-59
22.. Tamboli P, Toprani TH, Amin MB, Carcinoma of lung with rhabdoid features: Hum Pathol, 2004; 35; 8-13
23.. Yilmazbayhan D, Ates LE, Dilege S, Pulmonary large cell carcinoma with rhabdoid phenotype: Ann Diagn Pathol, 2005; 9; 223-26
24.. Falconieri G, Moran CA, Pizzolitto S, Intrathoracic rhabdoid carcinoma: A clinicopathological, immunohistochemical, and ultrastructural study of 6 cases: Ann Diagnos Pathol, 2005; 9; 279-83
25.. Goto H, Ito M, Yamaguchi N, [A case of large cell carcinoma of the lung with rhabdoid phenotype]: Nihon Kokyuki Gakkai Zasshi, 2006; 44; 325-29 [in Japanese]
26.. Song DE, Jang SJ, Black J, Ro JY, Mucinous bronchioloalveolar carcinoma of lung with a rhabdoid component – report of a case and review of the literature: Histopathology, 2007; 51; 427-30
27.. Saini G, Kumar M, Julka PK, Rhabdoid variant of lung cancer: Clinicopathological details of a case and a review of literature: J Cancer Res Ther, 2009; 5; 54-57
28.. Izquierdo-Garcia FM, Moreno-Mata N, Herranz-Aladro ML, Lung carcinoma with rhabdoid component a series of seven cases associated with uncommon types of non-small cell lung carcinomas and alveolar entrapment: Histol Histopathol, 2010; 25; 1287-95
29.. Otera H, Ikeda F, Nakagawa S, Intussusception of small intestine due to metastasis of large cell carcinoma of the lung with a rhabdoid pheno-type: Eur Respir Rev, 2010; 117; 248-52
30.. Attia A, Suleman M, Mosleh H, Malignant rhabdoid tumor of the lung in the young adult: A case report: Case Rep Pulmonol, 2011; 2011; 332584
31.. Dettmer M, Hench J, Pang B, Rhabdoid large cell carcinoma of lung, with illustrative immunohistochemical and molecular findings: Appl Immunohistochem Mol Morphol, 2012; 20; 208-13
32.. Kim MW, Rew SJ, Eun SJ, A case of lung carcinoma with rhabdoid phenotype mimicking an aspergilloma in patient with recurrent hemoptysis: Tuberc Respir Dis, 2014; 77; 38-41
33.. Bahadur S, Pujani M, Jetley S, Large cell lung carcinoma with rhabdoid phenotype: Report of a rare entity presenting with chest wall involvement: J Cancer Res Ther, 2015; 11; 657
34.. Zysman M, Clement-Duchene C, Bastien C, Malignant rhabdoid tumor of the lung: Rev Mal Respir, 2016; 33; 808-11
35.. : Thoracic Tumours WHO Classification of Tumours, 2021; 5
36.. Yang SR, Chang JC, Leduc C, Tan KS, Invasive mucinous adenocarcinomas with spatially separate lung lesions: Analysis of clonal relationship by comparative molecular profiling: J Thorac Oncol, 2021; 16; 1188-99
37.. Brabletz T, Kalluri R, Nieto MA, Weinberg RA, EMT in cancer: Nat Rev Cancer, 2018; 18; 128-34
38.. Suda K, Tomizawa K, Fujii M, Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib: J Thorac Oncol, 2011; 6; 1152-61
39.. Arner EN, Du W, Brekken RA, Behind the wheels of epithelial plasticity in KRAS-driven cancers: Front Oncol, 2019; 9; 1049
40.. Shao DD, Xue W, Krall EB, KRAS and YAP1 converge to regulate EMT and tumor survival: Cell, 2014; 158; 171-84
41.. Stewart BD, Kaye F, Machuca T, SMARCA4-deficient thoracic sarcoma: A case report and review of literature: Int J Surg Pathol, 2020; 28; 102-8
42.. Agaimy A, Fuchs F, Moskalev EA, SMARCA4-deficient pulmonary adenocarcinoma: Clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1neg/ CK7pos/HepPar-1pos immunophenotype: Virchows Arch, 2017; 471; 599-609
43.. Drilon A, Schoenfeld AJ, Arbour KC, Exceptional responders with invasive mucinous adenocarcinomas: A phase 2 trial of bortezomib in patients with KRAS G12D-mutant lung cancers: Cold Spring Harb Mol Case Stud, 2019; 5; a003665
44.. Italiano A, Soria JC, Toulmonde M, Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study: Lancet Oncol, 2018; 19; 649-59
45.. MacMahon H, Naidich DP, Goo JM, Guidelines for management of incidental pulmonary nodules detected on CT Images: from the Fleischner Society 2017: Radiology, 2017; 284; 228-43
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250