27 August 2021: Articles 
Ovarian Leydig Cell Tumor: Cause of Virilization in a Postmenopausal Woman
Challenging differential diagnosis, Rare disease
Nádia Mourinho Bala1BEF*, José Maria Aragüés1E, Sílvia Guerra1E, Delfina Brito2E, Cristina Valadas1EDOI: 10.12659/AJCR.933126
Am J Case Rep 2021; 22:e933126
Abstract
BACKGROUND: Only 0.5% of all ovarian tumors are Leydig cell tumors and they are generally benign and unilateral. These androgen-secreting tumors lead to virilizing symptoms, most often in postmenopausal women. Because Leydig cell tumors are typically small, diagnosing them accurately can be challenging.
CASE REPORT: We report the case of a 77-year-old woman who was referred to our Endocrinology Clinic because of a 5-year history of hirsutism (Ferriman-Gallwey score of 11) with no discernible cause. The patient had high levels of serum testosterone and a normal level of dehydroepiandrosterone sulfate. Imaging, including transvaginal ultrasound and pelvic magnetic resonance, revealed a 16-mm uterine nodule, which was suspected to be a submucous leiomyoma, but no adrenal or ovarian lesions. Despite the lack of findings on imaging and because of the high suspicion for an androgen-secreting ovarian tumor, bilateral laparoscopic oophorectomy was performed. Histological examination of the specimen revealed a non-hilar Leydig cell tumor that measured 8 mm in its largest axis. After the surgery, the patient had significant clinical improvement and her laboratory test results normalized. Her sister had the same symptoms and laboratory findings at a similar age, which raised the suspicion of a possible familial genetic syndrome. No genetic testing was performed, however, because the patient’s sister declined further diagnostic investigation.
CONCLUSIONS: Leydig cell tumors are rare, and even when they are small, they can cause symptoms related to androgen excess. As a result, diagnosing them often is challenging.
Keywords: hyperandrogenism, Leydig Cell Tumor, Postmenopause, Female, Humans, Ovarian Cysts, Ovarian Neoplasms, Virilism
Background
The appearance of a few terminal hairs on the face and a decrease in body and scalp hair in women during menopause are considered part of the natural process following cessation of menses [1,2]. The appearance of virilizing features in a menopausal patient, such as male alopecia, clitoromegaly, a deep voice, acne, increased libido, and hirsutism, however, should lead to an investigation. These symptoms can be due to adrenal tumors, including androgen-secreting carcinomas, and ovarian tumors. Leydig cell tumors are rare ovarian neoplasms classified as sex cord-stromal tumors and they can cause hyperandrogenism. Only 0.5% of all ovarian tumors are Leydig cell tumors and most are benign and unilateral [3]. Diagnosing them can be challenging because they may be symptomatic despite their small size and they often are not detected on imaging.
Case Report
We report the case of a 77-year-old woman with a history of autoimmune hepatitis, type 2 diabetes, dyslipidemia, and Hashimoto thyroiditis. She was taking azathioprine (50 mg bid) and was not receiving any treatment associated with hirsutism, including glucocorticoids. She had a history of 4 un-complicated pregnancies and experienced menopause at age 42 years. She had not taken hormone replacement therapy.
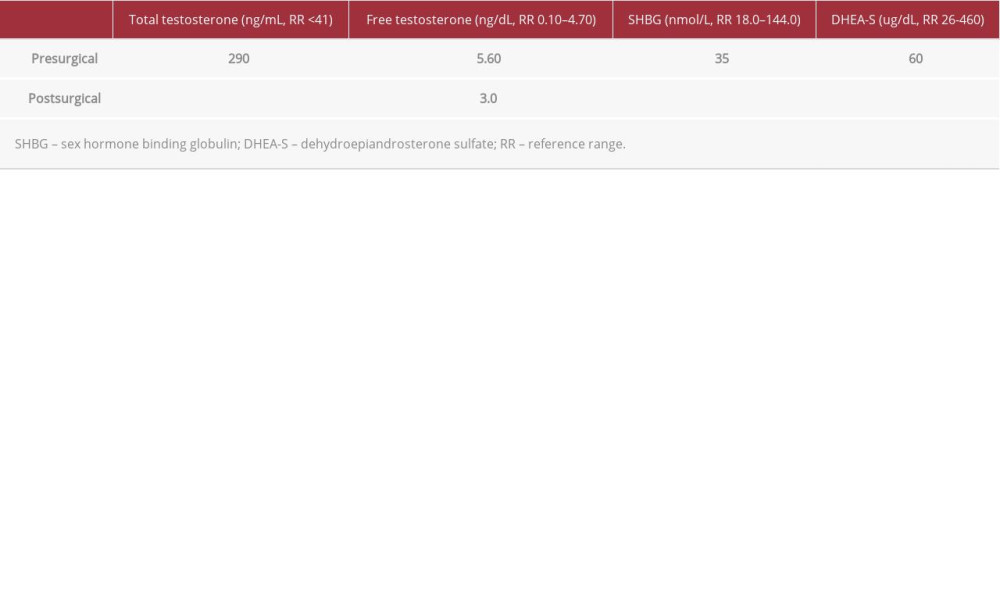
Over the past 4 years, the patient had experienced generalized hirsutism, which was most prominent on her face (Ferriman-Gallwey score of 11), and caused her significant psychological distress. Laboratory testing revealed the following levels: total testosterone 290 ng/dL (normal range, <41), free testosterone 5.60 ng/mL (normal range, 0.10–4.70), delta-4 androstenedione 2.9 ng/mL (normal range, 0.4–3.7), 17-hydroxyprogesterone 1.80 ng/mL, dehydroepiandrosterone sulfate (DHEA-S) 72 ug/dL (normal range, 26–460), thyroid stimulating hormone 2.84 mUI/L (normal range, 0.27–4.20), and free T4 16.0 pmol/L (normal range, 12.0–22.0). On transvaginal ultrasound (TVUS), after adjustment for age, the patient’s ovaries were enlarged but the echostructure was normal and there were no other findings. Pelvic magnetic resonance imaging (MRI) revealed only a 16-mm uterine nodule, which appeared to be a submucous leiomyoma, and no adrenal lesions.
After adrenal causes of virilization were eliminated, a laparoscopic bilateral oophorectomy and hysterectomy were performed. Histopathology revealed an 8-mm ovarian tumor with foci of luteinized cells and Reinke crystals, which was positive for inhibin and calretinin and negative for estrogen and progesterone receptors, findings that supported the diagnosis of a non-hilar Leydig cell tumor (Figure 1). After the surgery, the patient’s testosterone and free testosterone levels returned to normal (Table 1).
It is important to note that the patient’s sister had the same symptoms of marked hirsutism, at age 79 years. Her serums levels were as follows: total testosterone 258 ng/dL (normal range, <41), free testosterone 3.30 ng/mL (normal range, 0.10–4.70), serum hormone binding globulin 61.0 nmol/L (normal range, 18.0–144.0), DHEA-S 84 ug/dL (normal range, 26–460), and delta-4 androstenedione 3.1 ng/mL (normal range, 0.3–3.7). The patient’s sister underwent pelvic MRI, which showed normalsized ovaries and no large lesions. A bilateral oophorectomy was recommended, but she declined the treatment.
Discussion
Accurately diagnosing virilizing ovarian tumors is often challenging because they may be too small to detect on imaging. Identifying small functioning can require use of multiple types of imaging and an integrated approach. TVUS is often used as a good first-line exam and pelvic MRI adds to clear characterization of the ovarian anatomy. However, even both types of imaging together can be insufficient if a tumor is very small.
In the patient in our case, a normal serum DHEA-S level excluded adrenal causes of hyperandrogenism, leaving an ovarian androgen-secreting tumor as the most likely diagnosis. For this reason, an oophorectomy was recommended. After the surgery, the patient’s symptoms improved and her serum testosterone levels normalized. The histopathological examination supported the diagnosis of a non-hilar Leydig cell tumor, which is considered a steroid cell tumor. Such tumors are defined as ovarian neoplasms, composed of steroid hormone-secreting cells, and divided into 3 subtypes: stromal luteomas arising from ovarian stroma, Leydig cell tumors arising from Leydig cells in the hilum, and steroid cell tumors not otherwise specified when the lineage of the tumor is unknown [4]. This latter subtype is the most common, accounting for 80% of steroid cell tumors [5,6]. Usually, Leydig cell tumors arise from the ovarian hilum. Non-hilar Leydig cell tumors, as in our patient, occur in ovarian cortical stroma and can be difficult to distinguish from other stromal tumors [7].
Pure Leydig cell tumors of the non-hilar type closely resemble the stromal luteoma, a distinctive form of lipid cell tumor described by Scully [8]. It is distinguished from a stromal luteoma by the presence of characteristic crystalloids of Reinke within the tumor cells [8,9]. These tumors secrete androgens, which are responsible for virilizing symptoms, and they are usually unilateral and benign. However, there is a risk of malignant transformation. About 20% of patients develop metastasis, usually limited to the peritoneal cavity; metastasis to distant sites is rare [10]. Because the patient’s sister had the same symptoms and laboratory findings at a similar age, we suspected a familial genetic syndrome. However, no histologic diagnosis was confirmed for her because she declined surgery and genetic testing was not performed. In her case, therapy with spironolactone was provided.
Recent data indicate that germline or somatic mutations in some genes can lead to the development of some types of sex cord-stromal ovarian tumors [11]. DICER1 syndrome, which includes pleuropulmonary blastoma, thyroid gland neoplasia, pulmonary cysts, and cystic nephromas and is associated with Sertoli-Leydig tumors, is caused by a mutation in the
Conclusions
Leydig cell tumors are rare, challenging to diagnose, and should be considered in the differential for virilizing symptoms in postmenopausal women. The diagnosis is based on clinical history, laboratory findings, and imaging. After an adrenal cause of hyperandrogenism has been excluded, even in the absence of ovarian lesions, oophorectomy should be considered.
References:
1.. Alpañés M, González-Casbas JM, Sánchez J, Management of post-menopausal virilization: J Clin Endocrinol Metab, 2012; 97(8); 2584-88
2.. Thomas PK, Ferriman DG, Variation in facial and pubic hair growth in white women: Am J Phys Anthropol, 1957; 15(2); 171-80
3.. Lantzsch T, Stoerer S, Lawrenz K, Sertoli-Leydig cell tumor: Arch Gynecol Obstet, 2001; 264(4); 206-8
4.. Hayes MC, Scully RE, Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases: Am J Surg Pathol, 1987; 11(11); 835-45
5.. Outwater EK, Wagner BJ, Mannion C, Sex cord-stromal and steroid cell tumors of the ovary: Radiographics, 1998; 18(6); 1523-46
6.. Jiang W, Tao X, Fang F, Benign and malignant ovarian steroid cell tumors, not otherwise specified: Case studies, comparison, and review of the literature: J Ovarian Res, 2013; 6; 53
7.. Rabban JT, Soslow RA, Zaloudek CZ, Chapter 16 – Immunohistology of the female genital tract: Diagnostic Immunohistochemistry, 2010; 690-762
8.. Scully RE, Stromal luteoma of the ovary. A distinctive type of lipoid-cell tumorr: Cancer, 1964; 17(6); 769-78
9.. Sternberg WH, Roth LM, Ovarian stromal tumors containing leydig cells: Cancer, 1973; 32(4); 940-51
10.. Apelt-Alcalay D, Reyna-Villasmil E, Pure Leydig cell tumor of the ovary in a premenopausal woman: Rev Peru Ginecol Obs, 2020; 66(2); v66 i2257
11.. Macut D, Ilić D, Mitrović Jovanović A, Bjekić-Macut J, Androgen-secreting ovarian tumors: Front Horm Res, 2019; 53; 100-7
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250