18 October 2021: Articles 
Hypoplastic Left Atrial Appendage: A Case Report and Literature Review
Mistake in diagnosis, Diagnostic / therapeutic accidents, Rare coexistence of disease or pathology
Hitomi Hasegawa1ABCE, Takahide Ito1ACDEF*, Ryoto Hourai1BD, Kanako Akamatsu1CDE, Yoshifumi Nomura1D, Masatoshi Miyamura1AD, Shu-ichi Fujita1D, Masaaki Hoshiga1DDOI: 10.12659/AJCR.933260
Am J Case Rep 2021; 22:e933260
Abstract
BACKGROUND: The left atrial appendage (LAA) has considerable variations in its size, shape, and spatial relationship with other cardiac structures. Absence of the LAA is a congenital cardiac condition usually identified by an imaging modality intended for other purposes. Absence of the LAA has been described in a total of 19 case reports so far; however, no cases of “hypoplastic” LAA in a real sense have ever been reported.
CASE REPORT: We herein report a case of hypoplastic, but not truly absent, LAA in a 76-year-old man scheduled for catheter ablation against atrial flutter. Preprocedural transesophageal echocardiography (TEE) performed in this patient to exclude intracardiac thrombosis failed to detect the LAA, although Doppler color-flow mapping revealed a jet signal spewed out into the main LA around where the LAA would be located. The LAA was also not detectable by routinely developed tomographic images from computed tomography (CT) angiography. Eventually, however, the multiplanar reconstruction into 3-dimensional volume rendering via the CT angiography identified a very small LAA. Those findings by TEE and CT led to a diagnosis of hypoplastic LAA.
CONCLUSIONS: Hypoplastic LAA should be kept in mind when considering LAA interventions as well as anticoagulation treatment. Multiple imaging modalities are necessary to recognize morphological aberration of the LAA.
Keywords: Atrial Appendage, Atrial Fibrillation, Catheter Ablation, computed tomography angiography, Echocardiography, Transesophageal, Heart Defects, Congenital, Humans, Male
Background
The left atrial appendage (LAA) has considerable variations in its size, shape, and spatial relationship with other cardiac structures [1,2]. It has been reported to exert a beneficial effect on cardiac hemodynamics [3–5], although it is the most common site of atrial thrombus formation [6]. Now that the era of novel cardiac interventions such as LAA occlusion as well as newly introduced anticoagulation treatment have been developed [1,6], more enhanced understanding of LAA morphology may be required. A total of 19 cases of absence of LAA were identified in the literature up to March 2021. To the best of our knowledge, however, no cases of “hypoplastic” LAA in a real sense have ever been reported. We herein describe such a case of hypoplastic LAA, which was identified incidentally during transesophageal echocardiography (TEE) prior to catheter ablation for atrial arrhythmia. This case report also provides a short review of the clinical features for the 19 cases of so-called absence of the LAA.
Case Report
The patient was a 76-year-old man who had been treated for diabetes and dyslipidemia for the past decade. He began to notice shortness of breath on exertion 1 month before the current admission. Around the same time, he was found to have atrial flutter on an electrocardiogram at his annual check-up. As it became more symptomatic, he was referred to our hospital to undergo radiofrequency catheter ablation to treat this arrhythmia.
Upon admission, his temperature was 36.5°C, pulse rate was 90 beats/min (irregular), and blood pressure was 123/76 mmHg, and his oxygen saturation on room air was 97%. He looked well and normal, neither anemic nor icteric. The physical examination was not remarkable except for the irregular pulse. On cardiac auscultation, no extra sound or murmur was audible. The electrocardiogram showed atrial flutter, with typical saw-edged waves in the limb leads (II, III, aVF), and a relatively controlled heart rate (88 beats/min). The chest X-ray showed a slightly enlarged cardiac silhouette (cardiothoracic ratio 52%) without pulmonary congestion. The patient had a slight elevation of B-type natriuretic peptide level (43.9 pg/ mL), and no other abnormalities on the peripheral blood count or chemistry were found. The echocardiogram demonstrated no abnormal measurements including the left atrial (LA) diameter (36 mm) and left ventricular ejection fraction (66%), without evidence of underlying cardiac disorders such as cardiomyopathy or valvular disease. With a CHADS2 score calculated as 2, the patient began to receive anticoagulation treatment with edoxaban at a dosage of 30 mg/d.
TEE was performed to exclude intracardiac thrombosis, but the LAA, which is the most common site of thrombus formation, could not be detected (Figure 1A), even with the real-time 3-dimensional (3D) mode (Figure 1B). Doppler color-flow mapping, however, revealed a jet signal spewed out into the main LA from where the LAA might be located (Figure 2A). Moreover, with the pulsed Doppler sample volume placed at the jet signal, a flow-velocity pattern specific to that of an LAA was recorded (Figure 2B). No thrombotic lesions were found in any other cardiac chambers.
Findings from CT angiography were consistent with those from TEE. The LAA was also not detectable by routinely developed tomographic images (Figure 3A). The multiplanar reconstruction into 3D volume rendering, however, revealed a very small, stalk end-like LAA (Figure 3B). Those findings by TEE and CT in this patient led to a diagnosis of hypoplastic LAA. The catheter ablation was finished successfully, and he has continued to do well with regard to sinus rhythm without anticoagulation treatment.
Discussion
Within the first 4 weeks of embryonic life, the LAA develops from the primordial LA, which is formed through the adsorption of the primordial pulmonary veins [1,6,7]. The LAA shows a finger-like structure arising from the anterolateral site of the main LA [1]. The role of the LAA has not been elucidated sufficiently, but it has been suggested that the LAA assists LA contractile function [5,6] and also prevents an increase in LA pressure because of the LAA being more flexible than the main LA [4]. Nevertheless, the LAA has considerable clinical importance as the most common site of thrombus formation leading to thromboembolic events [6].
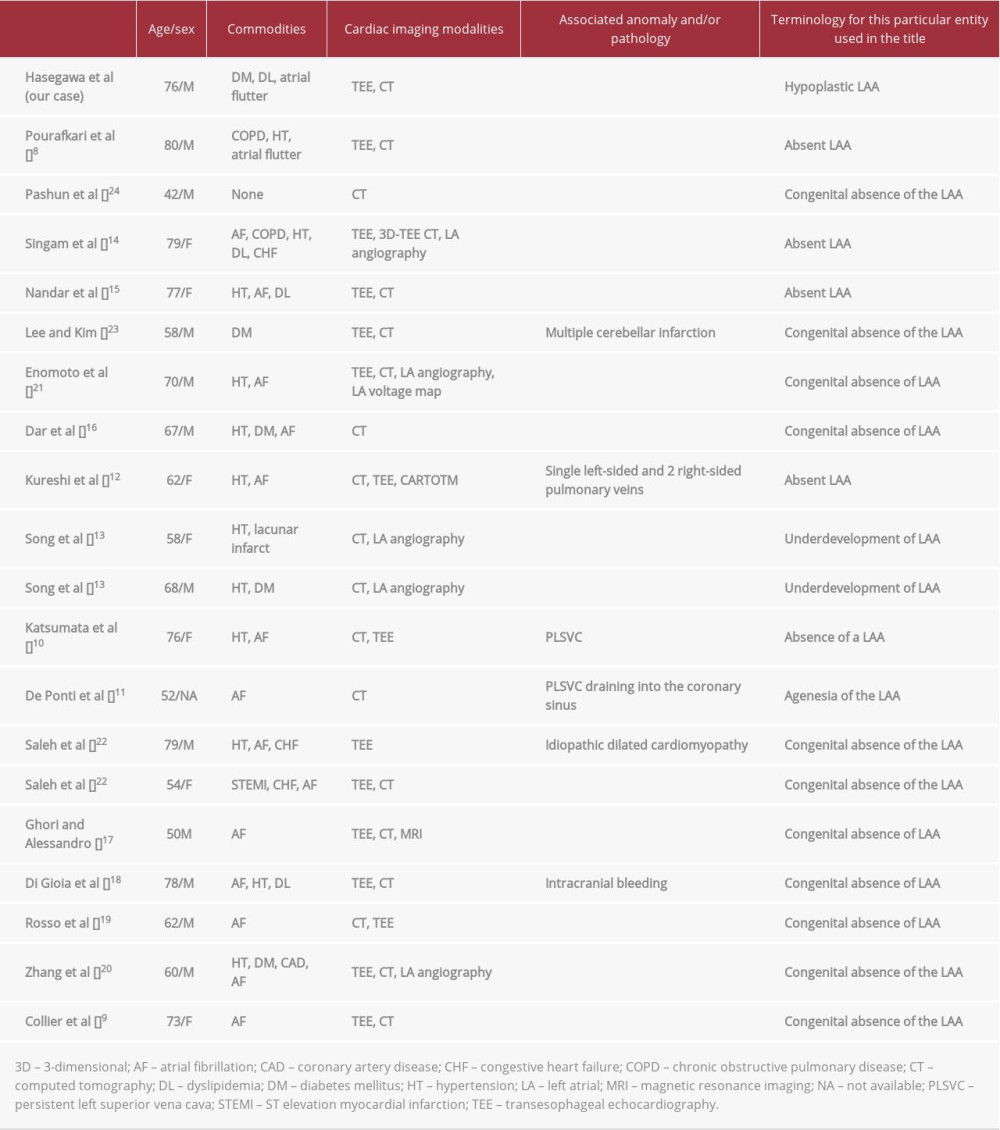
To the best of our knowledge, 19 cases of so-called absence of the LAA have been reported in the literature (in English) [8–24], with Collier et al [9] having described the first case in 2012 (Table 1). In most cases, such abnormal conditions are found incidentally during TEE or CT prior to catheter ablation for atrial fibrillation. A few cases were accompanied by persistent left superior vena cava as another congenital cardiac association documented by CT angiography [10,11]. Among the 19 case reports, 2 used “underdevelopment of the LAA” and “agenesia of the LAA” in their titles as a synonym for “absence of LAA” (Table 1) [11,13]. In this regard, our patient might be the first reported case describing truly hypoplastic LAA. Unlike any other reported cases, the LAA in our patient was located close to where it should be, albeit looking abnormal and similar to a stalk end, as demonstrated by 3D CT angiography (Figure 3B). The specific LAA morphology as well as its relationship with other cardiac structures might render the LAA as missing on the tomographic images.
In either morphological aberration of the LAA, multiple imaging modalities may be necessary to establish the diagnosis. In most previous cases, TEE and CT played a pivotal role in diagnosing such a condition. Song et al [13] and Zhang et al [20] used another imaging tool, LA angiography, in combination with TEE or CT, which completely delineated the absent LAA. Kureshi et al [12] and Enomoto et al [21] introduced the LA voltage map to examine whether this anomaly was related to abnormal potential or low voltage substance around where the LAA would exist. In the case we present, it should be emphasized that Doppler echocardiography on TEE gave a clue that enabled diagnosis of the hypoplastic LAA after detection by 2D and 3D TEE failed. Without the Doppler interrogation, this condition might have been overlooked (Figure 2).
Total thrombotic occlusion of the LAA should be taken into consideration as a differential diagnosis [22]. It might be critical in a case of thromboembolism with an unknown origin, given that an absent LAA was detected mostly among patients who have atrial fibrillation. A report from Korea presented such a case complicated by multiple cerebellar infarctions, and the authors suggested that anticoagulation treatment should be directed to atrial fibrillation patients even in the absence of the LAA [23].
Theoretically speaking, the risk of thromboembolism is relatively low in the absence of LAA due to the lack of an anatomical source of thrombus formation. Thus, anticoagulation treatment for the absence of the LAA may not be required. On the other hand, a hypoplastic LAA is considered to be associated with a certain risk of thromboembolism, for which the LAA flow velocity profile could guide decision-making on anticoagulation treatment [1]. However, given that the absence of the LAA or hypoplastic LAA is an extremely rare condition, a clinical trial on anticoagulation and subsequent prognosis is unlikely. An LAA occlusion procedure (Watchman device implantation) has recently been developed as an alternative treatment to atrial fibrillation in patients who do not tolerate long-term anticoagulation. Although the LAA occlusion procedure has been associated with a favorable clinical outcome compared with warfarin [25], it is not currently applied to patients with hypoplastic LAA due to the LAA not being large enough for the Watchman device.
Conclusions
We have reported a case of hypoplastic LAA incidentally found by preprocedural TEE. This entity was confirmed by TEE and 3D CT in combination. Most of the previous cases similar to ours described a truly absent LAA, implying an influence on decision-making for anticoagulation treatment and LAA interventions. To avoid missing its diagnosis, multiple imaging modalities should be introduced.
Figures
References:
1.. Beigel R, Wunderlich NC, Ho SY, The left atrial appendage: Anatomy, function, and noninvasive evaluation: JACC Cardiovasc Imaging, 2014; 7; 1251-65
2.. Kamiński R, Kosiński A, Brala M, Variability of the left atrial appendage in human hearts: PLoS One, 2015; 10; e0141901
3.. Hoit BD, Shao Y, Tsai LM, Altered left atrial compliance after atrial appendectomy: Influence on left atrial and ventricular filling: Circ Res, 1993; 72; 167-75
4.. Hoit BD, Shao Y, Gabel M, Influence of acutely altered loading conditions on left atrial appendage flow velocities: J Am Coll Cardiol, 1994; 24; 1117-23
5.. Ito T, Suwa M, Kobashi A, Influence of altered loading conditions on left atrial appendage function in vivo: Am J Cardiol, 1998; 81; 1056-59
6.. Patti G, Pengo V, Marcucci R, The left atrial appendage: From embryology to prevention of thromboembolism: Eur Heart J, 2017; 38; 877-87
7.. Al-Saady NM, Obel OA, Camm AJ, Left atrial appendage: Structure, function, and role in thromboembolism: Heart, 1999; 82; 547-54
8.. Pourafkari L, Sadeghpour A, Baghbani-Oskouei A, Absent left atrial appendage: Case report and review of the literature: Cardiovasc Pathol, 2020; 45; 107178
9.. Collier P, Cavalcante JL, Phelan D, Congenital absence of the left atrial appendage: Circ Cardiovasc Imaging, 2012; 5; 549-50
10.. Katsumata Y, Kashimura S, Nishiyama T, The absence of a left atrial appendage in a patient with paroxysmal atrial fibrillation with a persistent left superior vena cava: Eur Heart J Cardiovasc Imaging, 2017; 18; 374
11.. De Ponti R, Lumia D, Zoli L, Agenesia of the left atrial appendage: Possible but very rare: J Cardiovasc Med (Hagerstown), 2016; 17(Suppl. 2); e116-17
12.. Kureshi F, Bateman TM, Wimmer AP, The “absent” left atrial appendage: HeartRhythm Case Rep, 2017; 3; 494-95
13.. Song IG, Kim SH, Oh YS, Rho TH, Underdevelopment of left atrial appendage: Korean Circ J, 2017; 47; 141-43
14.. Singam NSV, Gopinathannair R, Stidam JM, A curious case of an absent left atrial appendage: Echocardiography, 2018; 35; 1882-84
15.. Nandar PP, Kichloo A, Aung TT, Kravitz KD, Therapeutic dilemma of natural watchman: Congenital absence of the left atrial appendage: Case Rep Cardiol, 2018; 2018; 7573425
16.. Dar T, Yarlagadda B, Swarup V, Lakkireddy D, Congenital absence of left atrial appendage: J Atr Fibrillation, 2017; 10; 1730
17.. Ghori MA, Alessandro S, Congenital absence of left atrial appendage: A case report and literature review: J Saudi Heart Assoc, 2015; 27; 132-34
18.. Di Gioia G, Mega S, Visconti S, Congenital absence of left atrial appendage in a patient with intracranial hemorrhage: Am J Case Rep, 2015; 16; 514-16
19.. Rosso R, Vexler D, Viskin S, Aviram G, Congenital absence of left atrial appendage: J Cardiovasc Electrophysiol, 2014; 25; 795
20.. Zhang ZJ, Dong JZ, Ma CS, Congenital absence of the left atrial appendage: A rare anatomical variation with clinical significance: Acta Cardiol, 2013; 68; 325-37
21.. Enomoto Y, Hashimoto G, Sahara N, Congenital absence of left atrial appendage diagnosed by multimodality imaging: Int Heart J, 2018; 59; 439-42
22.. Saleh M, Balakrishnan R, Castillo Kontak L, Congenital absence of the left atrial appendage visualized by 3D echocardiography in two adult patients: Echocardiography, 2015; 32; 1206-10
23.. Lee D, Kim D, Congenital absence of the left atrial appendage: An unexpected and incidental anomaly in a patient with multiple cerebellar infarctions: Chonnam Med J, 2018; 54; 133-34
24.. Pashun RA, Gannon MP, Tomassetti C, Congenital absence of the left atrial appendage: J Cardiovasc Comput Tomogr, 2020; 14; e115-17
25.. Price MJ, Safety and efficacy of transcatheter left atrial appendage closure for stroke prevention in patients with atrial fibrillation: Prog Cardiovasc Dis, 2018; 60; 542-49
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250