28 October 2021: Articles 
Hemodialysis Patient with Diffuse Liver Calcification After Septic Shock
Diagnostic / therapeutic accidents, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Kanako Hayashi12ABCDEF, Mineaki Kitamura12BDEF*, Hideshi Tomura12B, Kosei Yamaguchi12BDE, Tayo Kawazu1B, Kenji Sawase3B, Takashi Harada1ABCD, Tatsuki Ichikawa4D, Takahiro Takazono5BDE, Satoshi Funakoshi1BDE, Hiroshi Mukae5D, Tomoya Nishino2DDOI: 10.12659/AJCR.933386
Am J Case Rep 2021; 22:e933386
Abstract
BACKGROUND: Calcification in arteries is sometimes observed in patients undergoing hemodialysis; however, ectopic calcification in other organs is uncommon. In particular, diffuse liver calcification is very rare. We report a case of rapidly developing diffuse liver calcification in a patient undergoing hemodialysis.
CASE REPORT: An 82-year-old woman started hemodialysis because of diabetic nephropathy, and her renal function worsened due to acute coronary syndrome. Percutaneous coronary intervention was conducted, and she was referred to our hospital. However, she subsequently contracted various infections, including a urinary tract infection and pneumonia. On day 43 of hospitalization, she developed septic shock and liver dysfunction due to catheter-induced infection. Although she did not have any medical history of liver disease, hypoperfusion of the liver resulted in liver dysfunction, and a computed tomography scan conducted 3 months later showed diffuse calcification in her liver. Despite recovering from septic shock, she ultimately died of multiple organ failure 21 months after admission to our hospital.
CONCLUSIONS: Diffuse liver calcification is extremely rare; however, it can be observed in patients undergoing hemodialysis who experience liver hypoperfusion. The precise mechanisms underlying this disorder remain unknown, but a critically ill status and specific characteristics of hemodialysis patients may play important roles in liver calcification.
Keywords: Calcification, Physiologic, Renal Dialysis, renal insufficiency, Sepsis, Aged, 80 and over, Calcinosis, Female, Humans, Liver Diseases, Shock, Septic
Background
Calcification in arteries is not uncommon in patients undergoing hemodialysis and is accelerated by the systemic imbalance of minerals, including calcium and phosphate. Secondary hyper-parathyroidism has a negative effect on this mineral bone disorder and increases the risks of cardiovascular diseases partly via arterial calcification [1,2]. Since the formation of vascular lesions takes a long time, the prevalence of arterial calcification in patients with a long history of hemodialysis is higher than that in patients without it [3].
By contrast, ectopic calcification in other organs, such as muscles, is rare, apart from systemic calciphylaxis [4,5]. Although there have been reported cases so far, diffuse liver calcification is an extremely rare disorder; however, we experienced the case of a patient who developed diffuse liver calcification over a short period. This patient experienced severe septic shock repeatedly, and her computed tomography (CT) scan revealed diffuse liver calcification. This case differed from calciphylaxis in terms of localization in the liver and rapid progression of calcification. Thus, this case and other cases should be differentiated from calciphylaxis. Moreover, showing the clinical features of past cases and this case can be advantageous to elucidate the mechanism in patients undergoing hemodialysis with diffuse liver calcification. Herein, we report this case with a review of the literature.
Case Report
An 82-year-old woman with type 2 diabetes mellitus, who had been receiving diabetes treatment for more than 10 years, normally maintained her renal function with a serum creatinine concentration of 3 mg/dL. She had hypertension, and none of her relatives had renal diseases. Further, she was diagnosed with acute coronary syndrome and had pulmonary congestion, and percutaneous coronary intervention was performed at a general hospital. Thereafter, hemodialysis was initiated because her serum creatinine concentration increased to 7 mg/ dL, and she developed anuria. About 1 month later, she was referred to our hospital to continue treatment.
After admission to our hospital, she developed a urinary tract infection on day 10, and administration of 4.5 g of tazobactam piperacillin hydrate daily was initiated. In addition, she developed hypoxia on day 12. Her chest X-ray examination showed increased pleural effusion, and pneumonia was noted in the lower right lung field. From day 14, fasting and intravenous hyper-alimentation were started. Although her fever abated and X-ray examination showed improvement after day 18, she suddenly developed a fever (temperature, 38°C) on day 23, and a catheter-related infection was suspected. The central venous catheter was removed, and 0.5 g of vancomycin was administered after each hemodialysis session. Furthermore, her urine culture was positive for
Nonetheless, she again developed a fever (39°C) on day 38, and her systolic blood pressure dropped to 50 mmHg. Her serum C-reactive protein (CRP) level increased to 15 mg/dL, and her platelet count was 55 000/mm3 at that time. Due to shock, continuous venous dopamine was needed to maintain systemic blood flow. On day 43, her blood tests showed the following: aspartate aminotransferase (AST), 1832 U/L; alanine aminotransferase (ALT), 725 U/L; lactate dehydrogenase (LDH), 1348 U/L; alkaline phosphatase (ALP), 883 U/L; γ-glutamyl transpeptidase (γ-GTP), 98 U/L; procalcitonin increased to 37.8 ng/mL; and platelet count decreased to 17 000/mm3, suggesting the development of disseminated intravascular coagulation syndrome. Regarding chronic kidney disease-mineral bone disorders (CKD-MBD) parameters, serum-corrected calcium and serum phosphate levels were 8.8 mg/dL and 7.3 mg/dL, respectively, on day 43. Since the central venous catheter culture was positive for methicillin-resistant
Although her symptoms were improved by vancomycin, she developed high fever again on day 75. The intravenous catheter was removed, and vancomycin was restarted at the same dose as before, considering the exacerbation of MRSE infection. However, her blood culture was positive for
From day 112 to day 125, she had high fever (38.5°C). Teicoplanin (800 mg administered as a loading dose then 200 mg every 48-72 h to maintain a suitable concentration) and ceftazidime were administered despite her negative blood culture test result. Vancomycin could not be used because it caused skin eruption. Her blood test on day 131 revealed mild liver dysfunction: AST, 192 U/L; ALT, 132 U/L; LDH, 246 U/L; ALP, 922 U/L; and γ-GTP, 87 U/L. Regarding CKD-MBD parameters, her serum-corrected calcium level was 9.0 mg/ dL, serum phosphate level was 6.1 mg/dL, and iPTH level was 52 pg/mL on day 131. Although there were no abnormal liver findings on day 45 (Figure 1A), a second abdominal CT scan on day 136, approximately 3 months later, revealed faint and unclear high absorption areas in both lobes of the liver, indicating marked calcification (Figure 1B). In addition, her aorta showed slight calcification, which was not observed on day 45 (Figure 1B). Her overall clinical course until day 150 is summarized in Figure 2.
Although her liver function returned to normal, with enzyme levels maintained almost in the normal range, the patient repeatedly developed aspiration pneumonia, and
Discussion
Herein, we present the case of a hemodialysis patient with diffuse liver calcification. Because she repeatedly went into septic shock, ischemic liver perfusion and tissue injury seemed to have played a key role in the pathophysiology of liver calcification. In contrast, CKD-MBD parameters seemed to have little effect on the liver calcification.
Calcification is sometimes observed in various parts of the body, including the arteries, corneas, conjunctiva, periarterial tissue, skin, subcutaneous tissue, lungs, stomach, heart, and kidneys [6]. Arterial calcification is among the complications that most affect the prognosis of patients undergoing hemodialysis. Vascular calcification can be attributed to high serum Ca×P products, secondary hyperparathyroidism, lower serum Mg concentration, metabolic acidosis, and local tissue injuries. Apart from arterial calcification, uremic tumoral calcification and multiple organ calcification caused by systemic calciphylaxis have been observed among patients receiving dialysis [5,7]. Although the mechanisms underlying these disorders can differ, it takes a long time to develop calcification, irrespective of the etiology.
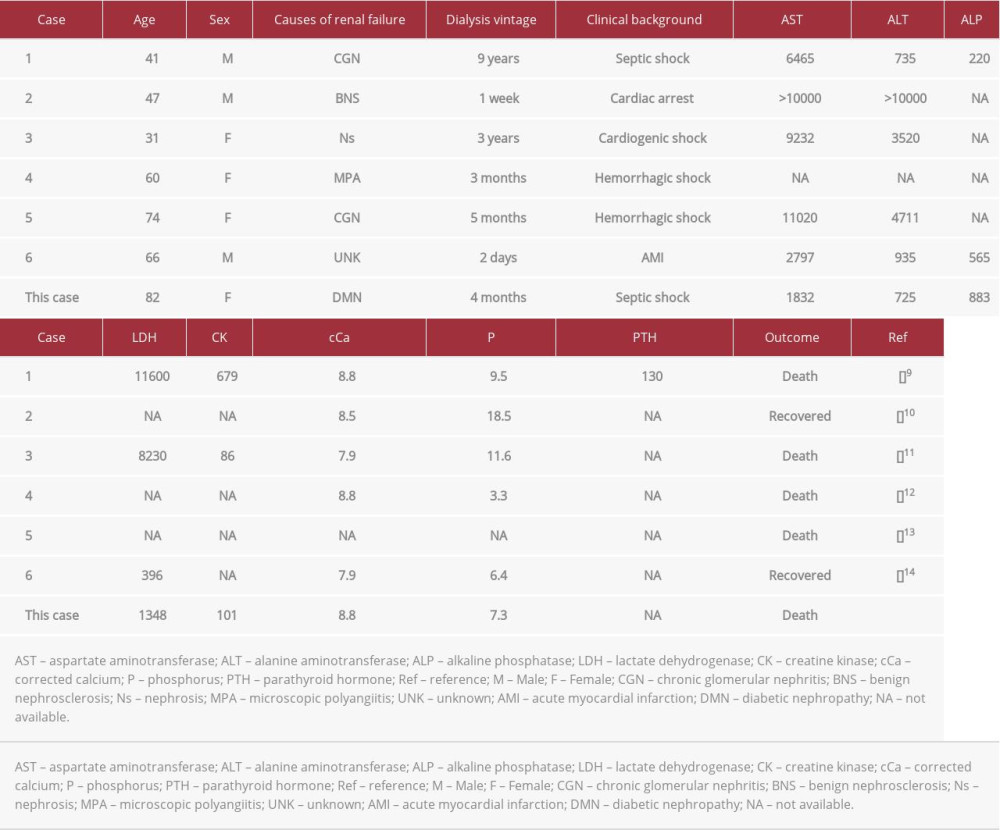
Liver tissue calcification is extremely rare, especially in patients undergoing hemodialysis. Generally, liver calcification is caused by granulomatous lesions, infections, echinococcosis, or malignancies [8]. Almost all reported cases showed local calcification along with vessel or interstitial calcification, and diffuse liver calcification was extremely rare [8]. In our search of medical databases, including MEDLINE and Google Scholar, we found only 6 previously reported cases [9–14] (Table 1). The prognosis of these patients tended to be poor, and all of these cases, as well as our case, were supposedly caused by liver ischemia due to systemic shock; this is called ischemic hepatitis or shock liver [15]. Regarding liver function, survivors in the previous cases and the present case showed the recovery of normal liver function because the liver is a tolerant organ with regenerative activity. The causes of this disorder vary and include heart failure, infection, and hypovolemic shock, and an acute liver injury is caused by insufficient oxygen delivery to the liver [15]. Some of these cases showed high Ca×P products, which might accelerate calcification; however, not all cases had poor control of mineral bone disorders. Regarding our patient, her serum phosphate level was high; however, the Ca×P products level was moderate. In addition, her serum iPTH level was low.
Additionally, our patient was not administered any vitamin D analogs throughout the clinical course, and calcium carbonate was not prescribed because her CKD-MBD parameters could be controlled without medications. Exogenous calcium intake should be considered; however, her intravenous hyper-alimentation or tube feeding contained only a small amount of calcium. In addition, the dialysate calcium level at our facility is within the range recommended by the 2017 Kidney Disease Improving Global Outcome CKD-MBD guidelines [16]. From this perspective, CKD-MBD parameters did not seem to play an important role in our patient’s developing liver calcification. In contrast, CKD-MBD might play a role in developing aortic calcification, as observed in the present case. A previous study suggested that ischemia of hepatocytes induces permeability of the cell membrane and causes calcium ions to flow into the hepatocytes, resulting in the activation of phospholipase. According to this hypothesis, absorption of phospholipids on the cellular membrane induces new calcium ion channels. After the restoration of blood flow, a large amount of calcium is absorbed into cells, which causes the disruption of mitochondria and enzyme and protein synthesis, finally leading to cell death [17].
Contrary to arterial calcification, diffuse liver calcification in the previously reported cases and the present case developed in a short period. Apart from cases 1 and 3, the previously reported cases and our case involved patients undergoing hemodialysis with a dialysis history of less than 6 months. We speculated that serum fetuin-A may be associated with liver calcification in patients who have been on hemodialysis for a shorter duration. Fetuin-A is known to prevent calcification; however, the serum fetuin level at the point of initiation of hemodialysis was proven to be low [18]. While continuing hemodialysis, the serum fetuin-A level gradually increased, suggesting that calcification may not be adequately prevented in patients who have just initiated hemodialysis. Moreover, serum parathyroid hormone levels are supposed to be higher at the initiation of hemodialysis [19], which might be associated with rapid liver calcification among patients who have just started hemodialysis.
Regarding fetuin-A, septic shock reduces serum fetuin-A level because inflammatory cytokines suppress the secretion of fetuin-A [20]. By contrast, the administration of fetuin-A significantly improved the survival rate in a septic animal model via high mobility group box protein 1 [21], suggesting that fetuin-A plays a key role in septic shock. Nevertheless, further molecular mechanisms need to be elucidated to clarify the progression of calcification.
This report has several limitations. First, liver biopsy was not performed owing to the poor general condition of the patient; moreover, an autopsy was not conducted. Therefore, details of microscopic change were not elucidated in this case. This is one of the most important limitations of this report. Second, the levels of liver enzymes increased in this case; however, not only ischemia but also antibiotics might be responsible for the liver dysfunction. Third, we did not perform an abdominal CT scan between days 45 and 136, that is, for approximately 3 months. Therefore, the precise speed at which the liver calcification developed remains unknown. Finally, the result of blood examinations was limited in this case, and her serum fetuin-A and serum 25-hydroxyvitamin D levels were not evaluated throughout the period.
We only speculate that lower serum fetuin-A level accelerates diffuse liver calcification; however, there was no evidence to support our hypothesis. Thus, the relationship between ectopic calcification and septic shock should be elucidated based on molecular biology techniques, including in animal models, in the future.
Conclusions
We report a rare case of a hemodialysis patient who repeatedly contracted urinary tract infection and pneumonia, resulting in septic shock. After her recovery from ischemia of hepatocyte, her liver showed diffuse calcification. Mineral imbalance and liver hypoperfusion could cause diffuse liver calcification in patients undergoing hemodialysis. Hence, we should pay careful attention to liver hypoperfusion in these patients to prevent lethal outcomes.
Figures
References:
1.. Cunningham J, Locatelli F, Rodriguez M, Secondary hyperparathyroidism: Pathogenesis, disease progression, and therapeutic options: Clin J Am Soc Nephrol, 2011; 6; 913-21
2.. Kakani E, Elyamny M, Ayach T, El-Husseini A, Pathogenesis and management of vascular calcification in CKD and dialysis patients: Semin Dial, 2019; 32; 553-61
3.. Ohtake T, Kobayashi S, Impact of vascular calcification on cardiovascular mortality in hemodialysis patients: Clinical significance, mechanisms and possible strategies for treatment: Ren Replace Ther, 2017; 3; 1-11
4.. Cozzolino M, Ciceri P, Ectopic calcification in uremia: Where do we stand: Blood Purif, 2020; 49; 641-42
5.. Hamada JI, Tamai K, Ono W, Saotome K, Uremic tumoral calcinosis in hemodialysis patients: Clinicopathological findings and identification of calcific deposits: J Rheumatol, 2006; 33; 119-26
6.. Floege J, When man turns to stone: Extraosseous calcification in uremic patients: Kidney Int, 2004; 65; 2447-62
7.. Ladino M, Sadhu S, Ortega LM, Calcification of the liver in a patient on renal replacement therapy diagnosed with systemic calciphylaxis: NDT Plus, 2009; 2; 504-5
8.. Patnana M, Menias CO, Pickhardt PJ, Liver calcifications and calcified liver masses: pattern recognition approach on CT: Am J Roentgenol, 2018; 211; 76-86
9.. Kinjo K, Yamashiro M, Akamine K, Diffuse hepatocellular calcification developing in a patient on chronic hemodialysis after ischemic hepatitis: Intern Med (Tokyo, Japan), 2007; 46; 1729-33
10.. Milstein MJ, Moulton JS, Diffuse hepatic calcification after ischemic liver injury in a patient with chronic renal failure: Am J Roentgenol, 1993; 161; 75-76
11.. Shibuya A, Unuma T, Sugimoto T, Diffuse hepatic calcification as a sequela to shock liver: Gastroenterology, 1985; 89; 196-201
12.. Sugiura H, Yoshida K, Nakanuma Y, Hepatic calcification in the course of hemodialysis: Am J Gastroenterol, 1987; 82; 786-89
13.. Morita R, Tateno M, Fujino S, A case of diffuse hepatocellular calcification in the patient with microscopic PN: Japanese J Diagnostic Pathol, 2017; 34; 106-8
14.. Kuji T, Takeda K, Watanabe Y, A case of liver calcification with chronic renal failure, after acute myocardial infarction: J Japanese Soc Dial Ther, 1996; 29; 425-28
15.. Soleimanpour H, Safari S, Rahmani F, Hepatic shock differential diagnosis and risk factors: A review article: Hepat Mon, 2015; 15; e27063
16.. Moe SM, Drueke TB, KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease mineral and bone disorder (CKD-MBD): Kidney Int, 2017; 76; S1-128
17.. Chien KR, Abrams J, Serroni A, Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury: J Biol Chem, 1978; 253; 4809-17
18.. Ponte B, Pruijm M, Pasch A, Dialysis initiation improves calcification propensity: Nephrol Dial Transplant, 2020; 35; 495-502
19.. Melamed ML, Eustace JA, Plantinga L, Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study: Kidney Int, 2006; 70; 351-57
20.. Karampela I, Kandri E, Antonakos G, Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: A prospective study: J Crit Care, 2017; 41; 78-85
21.. Li W, Zhu S, Li J, A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation: PLoS One, 2011; 6; 18-24
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250