30 October 2021: Articles 
Herpes Simplex Virus Meningoencephalitis Following Pulse-Dose Methylprednisolone: A Case Report and Literature Review
Unusual or unexpected effect of treatment
Jeffrey Horn1ABCDEF, Jon B. Mullholand1ABCDEF, Saad Ashraf1ABF, David Shore1ABCD, Andry Van de Louw1ABCDEF*DOI: 10.12659/AJCR.933847
Am J Case Rep 2021; 22:e933847
Abstract
BACKGROUND: Several cases of herpes simplex virus type 1 meningoencephalitis (HSVE) have been reported in patients receiving steroids, but the exact contribution of steroids to the disorder remains unclear because other risk factors, such as chemotherapy, brain radiation, or surgery, were present in almost all cases.
CASE REPORT: We report the case of a 76-year-old man who developed HSVE following the administration of pulse-dose steroids. The patient had occupational asbestos exposure and a chronic interstitial lung disease of unclear etiology (sarcoidosis versus hypersensitivity pneumonitis) and was admitted for acute-on-chronic respiratory failure requiring mechanical ventilation. After a negative infectious workup and several days of antibiotics without improvement, pulse-dose steroids were administered. In the following days, the patient developed a fever and worsening encephalopathy. A lumbar puncture showed elevated nucleated cells and positive polymerase chain reaction for herpes simplex virus 1 in the cerebrospinal fluid, confirming the diagnosis of HSVE. Acyclovir treatment was initiated, but the patient later died as a result of persistent severe encephalopathy and respiratory failure with an inability to wean mechanical ventilation.
CONCLUSIONS: Clinicians should keep in mind that HSVE is a potential complication of steroids and carefully consider the benefit/risk ratio of pulse-dose steroids, taking into account associated factors of immunosuppression. A high level of awareness should be especially maintained in critically ill patients because of associated risk factors (critical illness immune paralysis) and because neurological signs of HSVE may be missed in mechanically ventilated, sedated patients.
Keywords: Critical Care, Encephalitis, Herpes Simplex, Steroids, acyclovir, Herpesvirus 1, Human, Humans, Male, Meningoencephalitis, Methylprednisolone
Background
Herpes simplex virus (HSV) is the most common infectious cause of sporadic encephalitis worldwide, with HSV-1 being the most common strain to cause HSV encephalitis (HSVE) [1]. After primary mucocutaneous infection, HSV remains latent in sensory ganglia and 60–90% of the world’s population is estimated to be seropositive for HSV [1]. Possible pathogenic mechanisms for HSVE include reactivation of latent HSV in the trigeminal ganglia, with subsequent spread of infection to the temporal and frontal lobes, primary central nervous system (CNS) infection, and perhaps reactivation of latent virus within the brain parenchyma itself [1]. The introduction of acyclovir has decreased mortality rates from 70% to 15% [2], but early diagnosis and treatment initiation are major determinants of mortality [2].
Immunocompromised individuals seem to be at higher risk for severe HSV infections including meningoencephalitis [3], but the precise contribution of steroids remains unclear. Owing to their anti-inflammatory and immunomodulatory effects, glucocorticoids might in theory increase the rate of viral replication, but experimental animal models have yielded conflicting results regarding their effect on replication and clearance of HSV in the CNS [4,5]. Case reports have shown temporal associations between steroids and HSVE, but the relative contribution of steroids remains unclear because other factors, such as concomitant chemotherapy [6,7] or direct insult to the CNS (radiation [8], surgery [9,10]) or close structures (eye [11,12]), may have contributed to the HSVE in most of these cases. Here, we present a case of HSV-1 meningoencephalitis that developed following pulse-dose steroids for the treatment of interstitial lung disease in a patient with no history of chemotherapy or CNS insult. This case highlights the potential risk of pulse-dose steroids in patients with associated risk factors (age, critical illness immune paralysis, lymphopenia) and the need to rule out CNS infection in case of persistent, unexplained encephalopathy in critically ill patients. Informed consent for publication was obtained from the patient’s next of kin.
Case Report
A 76-year-old man was admitted with acute-on-chronic hypoxic respiratory failure. He had a past medical history of ischemic cardiomyopathy with reduced ejection fraction, atrial fibrillation, and ventricular tachycardia requiring a pacemaker and amiodarone, and he was on home oxygen owing to chronic hypoxic respiratory failure. The patient had a significant smoking history and occupational asbestos exposure. A year prior to admission he underwent the following procedures for dyspnea: (1) a pulmonary function test that showed normal spirometry, decreased lung volumes, and severely impaired diffusion capacity, consistent with restrictive lung disease; (2) a chest computed tomography (CT) scan that revealed perilymphatic nodular opacities (some calcified), ground glass opacities, and lower lobe predominant septal thickening; and (3) unre-markable bronchoscopy and broncho-alveolar lavage. A repeat chest CT a month prior to admission revealed worsening inter-stitial lung disease concerning for sarcoidosis versus chronic hypersensitivity pneumonitis. On admission, the patient was afebrile with a normal neurological exam. Significant laboratory values included white blood cell count 11.4×109/L (lymphocytes 0.66×109/L), procalcitonin 0.15 ng/mL, C-reactive protein 10.3 mg/dL, and brain natriuretic peptide 5374 pg/ mL. A chest CT showed no acute pulmonary embolus, but new ground glass and consolidative opacities were observed in both upper lobes. The patient was started on antibiotics for presumed community-acquired pneumonia and diuretics for heart failure exacerbation. He required intubation and mechanical ventilation on day 3 for worsening acute hypoxic respiratory failure. An extensive infectious workup, including 2 bronchoscopies with broncho-alveolar lavage, was entirely negative (bacterial and fungal cultures, multiplex polymerase chain reaction (PCR) for respiratory viral panel performed on a nasal swab and broncho-alveolar lavage fluids,
On day 13, the patient became febrile with worsening hyper-leukocytosis; urine culture was positive for
Within the next few days, attempts to wean sedation and mechanical ventilation were unsuccessful. The patient was intermittently following commands during sedation holidays, but he was very tachypneic with patient/ventilator dyssynchrony, requiring sedation to be maintained. On day 18, the patient was noted to be minimally responsive to commands, which was a worsening of his mental status from previous days. He was febrile again and his white blood cell count was 20.1×109/L. A head CT scan was unremarkable, but a brain MRI could not be obtained due to the pacemaker. A lumbar puncture was performed, and cerebrospinal fluid studies were significant for nucleated cells of 36/μL (92% neutrophils), red blood cells of 8000/μL, glucose 73 mg/dL (normal 40–70 mg/dL), protein 46 mg/dL (normal 15–45 mg/dL), and lactate 2.8 mmol/L (normal 1.1–2.4 mmol/L). Bacterial cultures were negative, but PCR results for HSV-1 were positive.
The patient was started on acyclovir 10 mg/kg every 8 h for HSV-1 meningoencephalitis. Steroids were tapered and eventually discontinued. The patient’s mental status did not improve, and severe patient/ventilator dyssynchrony persisted with an inability to wean mechanical ventilation. The patient was transitioned to comfort care and died on day 25. Of note, he had remained significantly lymphopenic during the entire Intensive Care Unit (ICU) stay (lymphocyte count consistently below 0.8×109/L with a nadir of 0.01×109/L) with a CD4 count of 66/mm3 and a CD4/CD8 ratio of 1.84. Screening for HIV and viral hepatitis yielded negative results.
An autopsy was performed, and significant lung findings included interstitial fibrosis most compatible with silicosis, pulmonary congestion, acute bronchopneumonia in the right lower lobe, and subacute infarction with necrosis and cavitation in left upper lobe. The neuropathology did not reveal any acute process.
Discussion
This case illustrates how important and challenging the discussion of the benefit/risk ratio is prior to administering pulse-dose steroids in critically ill patients. Steroids were administered in our patient, with the dosage subsequently being increased because of the lack of improvement after treatment of presumed pneumonia and heart failure exacerbation. The steroid treatment was also used because diagnoses of sarcoidosis or hypersensitivity pneumonitis were considered based on previous medical history and chest imaging. However, the autopsy ruled out these diagnoses.
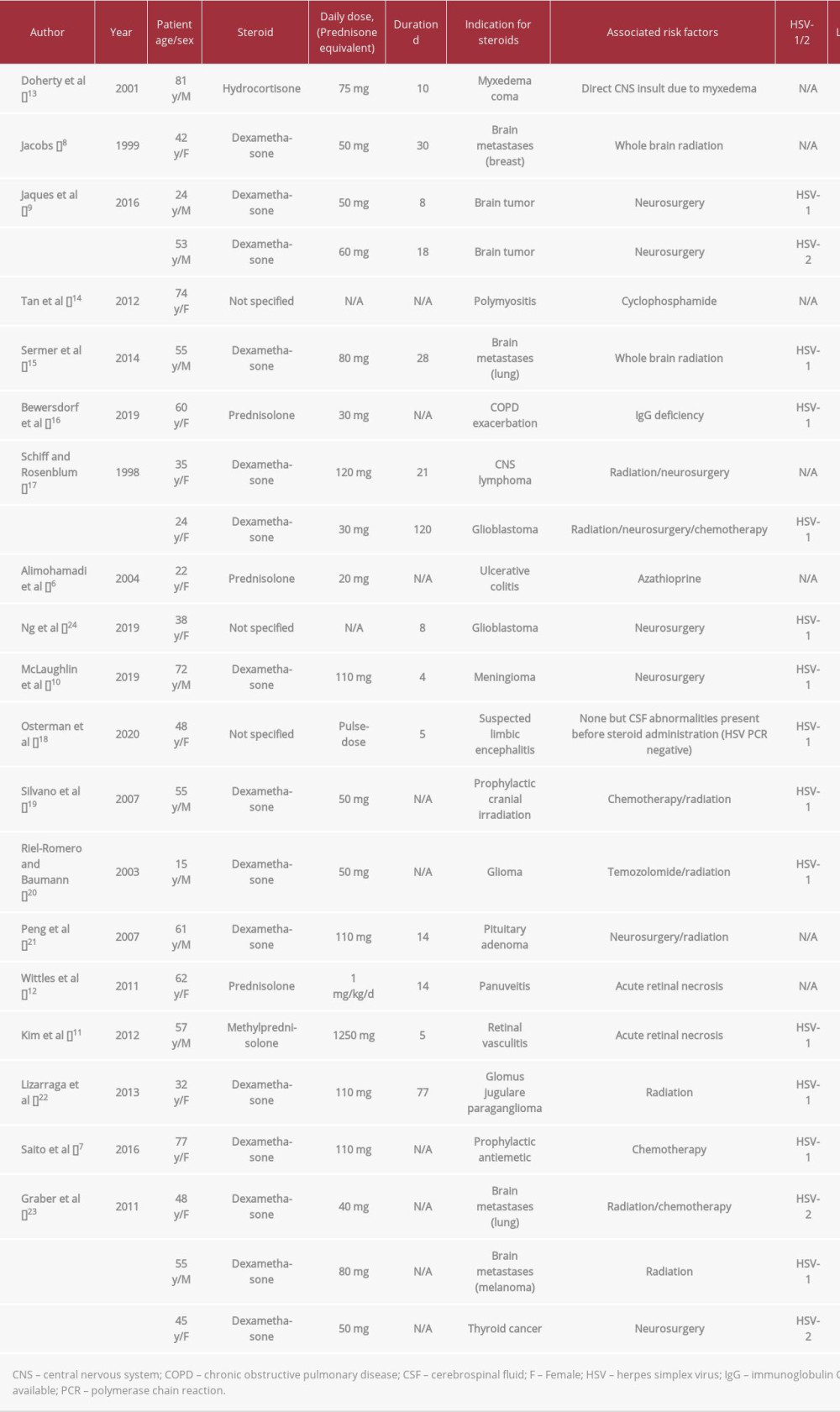
The risk of developing severe HSV infection after high-dose steroids is difficult to ascertain. In patients with systemic lupus erythematosus treated with various immunosuppressive drugs, the risk of severe HSV infection is increased [3]; steroids have been specifically associated with increased rates of opportunistic infections and herpes zoster infection in this population. The hypothesis of an increased risk of HSV infection due to steroids is therefore reasonable, but it has not been conclusively proven so far. Multiple cases of steroid-associated HSVE have been reported and are summarized in Table 1 [6–8,10–24]. In almost all cases, the presence of other potential triggers of HSVE, such as immunosuppressive drugs or direct CNS injury (surgery, radiation, eye injury), prevents any definite conclusion regarding a specific effect of steroids.
Our patient did not have a history of chemotherapy administration or direct CNS injury, but his lymphopenia on presentation was another potential risk factor for HSVE and makes a causal link between steroids and HSVE difficult to assert.
The lymphopenia consistently present in our patient throughout his admission and very low CD4 count may have indeed increased his risk of developing HSVE. Its cause remains unclear. HIV screening was negative, and the lymphopenia was ascribed on admission to possible sarcoidosis; however, the autopsy did not confirm that diagnosis. After inoculation of HSV in experimental models, antilymphocyte serum allowed the development of viremia and fatal meningoencephalitis [25]. Lymphopenia has been associated with increased risk of infection in general in patients with systemic lupus erythematosus [26], and HSVE complicating drug-induced lymphopenia has been reported [27]. Lymphopenia was present in our patient even before steroid administration, but steroids are known to decrease circulating lymphocyte count and function, especially T cells and the CD4 subset [28], and may have further altered our patient’s immune system.
Critical illness and sepsis per se are associated with lymphopenia, monocyte deactivation, and immune paralysis [29]. Lymphopenia in our patient was not secondary to sepsis because it was present prior to his admission. Pre-existing lymphopenia, critical illness-induced immune paralysis, and steroids may have synergistically led to the development of HSVE. However, despite a high prevalence of lymphopenia and immune paralysis in critically ill patients, regardless of the presence of sepsis [30], the occurrence of ICU-acquired HSVE is very rare [31] and its reality has even been challenged [32]. It is therefore likely that steroids played a key role in our patient even though a causal link with HSVE cannot be proven. Another concern specific to critically ill patients is that alarming neurological signs associated with HSVE may be missed in sedated, mechanically ventilated patients, which could cause delayed diagnosis and increased mortality. From that perspective, implementing daily interruption of sedation in these patients, which has been associated with better outcomes in randomized control trials [33,34], might also allow earlier detection of alarming signs, earlier diagnosis of HSVE, and improved outcome. The presence of fever, reported in 80–90% of patients with HSVE [35], should prompt a lumbar puncture when it is unexplained and associated with persistent altered mental status during sedation interruptions in immunocom-promised patients.
The fact that our patient also developed a
Conclusions
In summary, clinicians should keep in mind that HSVE is a potential complication of steroids and carefully consider the benefit/risk ratio of pulse-dose steroids in critically ill patients, taking into account factors associated with immuno-suppression. A high level of awareness should be especially maintained for critically ill patients because of associated risk factors (critical illness immune paralysis) and because neurological signs of HSVE may be missed in mechanically ventilated, sedated patients.
References:
1.. Bradshaw MJ, Venkatesan A, Herpes simplex virus-1 encephalitis in adults: Pathophysiology, diagnosis, and management: Neurotherapeutics, 2016; 13(3); 493-508
2.. Raschilas F, Wolff M, Delatour F, Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: Results of a multicenter study: Clin Infect Dis, 2002; 35(3); 254-60
3.. Li TH, Lai CC, Wang WH, Risk of severe herpes simplex virus infection in systemic lupus erythematosus: Analysis of epidemiology and risk factors analysis in Taiwan: Ann Rheum Dis, 2019; 78(7); 941-46
4.. Baringer JR, Klassen T, Grumm F, Experimental herpes simplex virus encephalitis. Effect of corticosteroids and pyrimidine nucleoside: Arch Neurol, 1976; 33(6); 442-46
5.. Thompson KA, Blessing WW, Wesselingh SL, Herpes simplex replication and dissemination is not increased by corticosteroid treatment in a rat model of focal herpes encephalitis: J Neurovirol, 2000; 6(1); 25-32
6.. Alimohamadi SM, Malekzadeh R, Mirmadjless SH, Herpes simplex virus encephalistis during immunosuppressive treatment of ulcerative colitis: MedGenMed, 2004; 6(4); 7
7.. Saito M, Kiyozaki H, Obitsu T, Herpes simplex virus-1 encephalitis induced by chemoradiotherapy and steroids in an esophageal cancer patient: A case report: BMC Cancer, 2016; 16; 233
8.. Jacobs DH, Herpes simplex virus encephalitis following corticosteroids and cranial irradiation: Neurology, 1999; 52(5); 1108-9
9.. Jaques DA, Bagetakou S, L’Huillier AG, Herpes simplex encephalitis as a complication of neurosurgical procedures: Report of 3 cases and review of the literature: Virol J, 2016; 13; 83
10.. McLaughlin DC, Achey RL, Geertman R, Grossman J, Herpes simplex reactivation following neurosurgery: Case report and review of the literature: Neurosurg Focus, 2019; 47(2); E9
11.. Kim SJ, Kang SW, Joo EY, An unusual case of herpes simplex viral encephalitis following acute retinal necrosis after administration of a systemic steroid: J Epilepsy Res, 2012; 2(1); 21-24
12.. Wittles KN, Goold LA, Gilhotra JS, Herpes simplex encephalitis presenting after steroid treatment of panuveitis: Med J Aust, 2011; 195(2); 87-88
13.. Doherty MJ, Baxter AB, Longstreth WT, Herpes simplex virus encephalitis complicating myxedema coma treated with corticosteroids: Neurology, 2001; 56(8); 1114-15
14.. Tan IL, McArthur JC, Venkatesan A, Nath A, Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised: Neurology, 2012; 79(21); 2125-32
15.. Sermer DJ, Woodley JL, Thomas CA, Hedlund JA, Herpes simplex encephalitis as a complication of whole-brain radiotherapy: A case report and review of the literature: Case Rep Oncol, 2014; 7(3); 774-79
16.. Bewersdorf JP, Koedel U, Patzig M, Challenges in HSV encephalitis: Normocellular CSF, unremarkable CCT, and atypical MRI findings: Infection, 2019; 47(2); 267-73
17.. Schiff D, Rosenblum MK, Herpes simplex encephalitis (HSE) and the immunocompromised: A clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection: Hum Pathol, 1998; 29(3); 215-22
18.. Osterman A, Ruf VC, Domingo C, Travel-associated neurological disease terminated in a postmortem diagnosed atypical HSV-1 encephalitis after high-dose steroid therapy – a case report: BMC Infect Dis, 2020; 20(1); 150
19.. Silvano G, Lazzari G, Resta F, A herpes simplex virus-1 fatal encephalitis following chemo-radiotherapy, steroids and prophylactic cranial irradiation in a small cell lung cancer patient: Lung Cancer, 2007; 57(2); 243-46
20.. Riel-Romero RM, Baumann RJ, Herpes simplex encephalitis and radiotherapy: Pediatr Neurol, 2003; 29(1); 69-71
21.. Peng T, Blakeley J, Cingolani E, Herpes simplex encephalitis in a patient with recurrent pituitary adenoma receiving radiation therapy: Am J Clin Oncol, 2007; 30(6); 664-65
22.. Lizarraga KJ, Alexandre LC, Ramos-Estebanez C, Merenda A, Are steroids a beneficial adjunctive therapy in the immunosuppressed patient with herpes simplex virus encephalitis?: Case Rep Neurol, 2013; 5(1); 52-55
23.. Graber JJ, Rosenblum MK, DeAngelis LM, Herpes simplex encephalitis in patients with cancer: J Neurooncol, 2011; 105(2); 415-21
24.. Ng S, Le Corre M, Aloy E, Herpes simplex encephalitis shortly after surgery for a secondary glioblastoma: A case report and review of the literature: World Neurosurg, 2019; 129; 13-17
25.. Zisman B, Hirsch MS, Allison AC, Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice: J Immunol, 1970; 104(5); 1155-59
26.. Ng WL, Chu CM, Wu AK, Lymphopenia at presentation is associated with increased risk of infections in patients with systemic lupus erythematosus: QJM, 2006; 99(1); 37-47
27.. Perini P, Rinaldi F, Puthenparampil M, Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient: Mult Scler Relat Disord, 2018; 26; 68-70
28.. Slade JD, Hepburn B, Prednisone-induced alterations of circulating human lymphocyte subsets: J Lab Clin Med, 1983; 101(3); 479-87
29.. Boomer JS, To K, Chang KC, Immunosuppression in patients who die of sepsis and multiple organ failure: JAMA, 2011; 306(23); 2594-605
30.. Hohlstein P, Gussen H, Bartneck M, Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis: J Clin Med, 2019; 8(3); 353
31.. Algahtani H, Shirah B, Hmoud M, Subahi A, Nosocomial herpes simplex encephalitis: A challenging diagnosis: J Infect Public Health, 2017; 10(3); 343-47
32.. Jouan Y, Grammatico-Guillon L, Guillon A, Nosocomial herpes simplex encephalitis: does it really exist?: J Infect Public Health, 2018; 11(1); 142
33.. Girard TD, Kress JP, Fuchs BD, Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial: Lancet, 2008; 371(9607); 126-34
34.. Kress JP, Pohlman AS, O’Connor MF, Hall JB, Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation: N Engl J Med, 2000; 342(20); 1471-77
35.. Whitley RJ, Herpes simplex encephalitis: Adolescents and adults: Antiviral Res, 2006; 71(2–3); 141-48
36.. Guery BP, Arendrup MC, Auzinger G, Management of invasive candidiasis and candidemia in adult non-neutropenic intensive care unit patients: Part I. Epidemiology and diagnosis: Intensive Care Med, 2009; 35(1); 55-62
37.. Dimopoulos G, Ntziora F, Rachiotis G: Anesth Analg, 2008; 106(2); 523-29
38.. Poissy J, Damonti L, Bignon A, Risk factors for candidemia: A prospective matched case-control study: Crit Care, 2020; 24(1); 109
39.. Spec A, Shindo Y, Burnham CA: Crit Care, 2016; 20; 15
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250