13 January 2022: Articles 
Acute Perforated Appendicitis Associated with Appendiceal Diverticulitis in a Young Man: A Case Report with Literature Review
Challenging differential diagnosis
Abdulrahim Ahmed Abdulmomen1AEF, Anwar Saeed AlZahrani1ADEF*, Liqa Abdulrahman Al Mulla2AEF, Faten Othman Alaqeel1AEFDOI: 10.12659/AJCR.934838
Am J Case Rep 2022; 23:e934838
Abstract
BACKGROUND: Diverticulosis of the vermiform appendix is rare. In patients who present with appendicitis, appendiceal diverticulitis as a cause due is also rare. We report the case of a 35-year-old man who presented with typical symptoms and signs of acute appendicitis, which was confirmed by histopathology to be due to perforated acute appendiceal diverticulitis.
CASE REPORT: A 35-year-old man presented to our Emergency Department with a 1-day history of right lower-quadrant abdominal pain that radiated to the left lower quadrant, which was associated with fever, vomiting, and abdominal distention. Biochemical analysis revealed mild leukocytosis. Computed tomography (CT) revealed signs of acute perforated appendicitis and early mass formation. The patient underwent laparoscopic appendectomy. Histopathological examination revealed appendiceal diverticulitis (pseudo-diverticulum).
CONCLUSIONS: Appendiceal diverticulitis is a rare surgical entity and is often an overlooked diagnosis. The differential diagnosis of appendiceal diverticulitis in patients presenting with signs of acute appendicitis is important as it is associated with a higher rate of complications such as perforation and an increased risk of appendiceal neoplasms. Appendectomy is a safe and appropriate treatment for appendiceal diverticulitis.
Keywords: Appendectomy, appendicitis, diverticulitis, Adult, Appendix, Humans, Intestinal Perforation, Male
Background
Appendiceal diverticulosis is an uncommon condition with a reported incidence of 0.014-1.9% [1]. It was first reported in 1893 by Kelynack and classified into 2 types, congenital and acquired, with the latter being more common [1,2]. The preoperative diagnosis of appendiceal diverticulitis (AD) is challenging as it mimics acute appendicitis on presentation [1]. However, this distinction is important because AD carries a higher risk of perforation and synchronous neoplasms, especially carcinoid tumors and mucinous adenomas, than does acute appendicitis [3,4]. Herein, we report the case of a 35-year-old man who presented with typical symptoms and signs of acute appendicitis, which was confirmed by histopathology to be due to perforated acute appendiceal diverticulitis.
Case Report
A 35-year-old Saudi man with no significant past medical history presented to the Emergency Department with a 1-day history of severe right lower abdominal pain associated with fever, multiple episodes of vomiting, and abdominal distension. The pain was continuous, progressive, and colicky in nature, and radiated to the left lower quadrant. The self-reported pain severity was 10/10. There was no prior history of similar episodes. His vital signs were normal and a physical examination revealed tenderness in the right lower quadrant, rebound tenderness, positive Rovsing’s and Dunphy’s signs.
Biochemical analysis showed leukocytosis (white blood cell count, 11 k/µL). An abdominal computed tomography (CT) scan revealed acute perforated appendicitis with early mass formation, with the appendix base arising from the base of the cecum medially posterior to the ileocecal valve with its tip coursing medially and superiorly. The body of the appendix was dilated, with at least 2 wall defects associated with small enhancing fluid loculations; the largest measured 0.9 cm while the smallest measured 0.4 cm. Peri-appendiceal fat stranding with thickening and enhancement of the adjoining peritoneal lining were noted, suggestive of focal peritonitis. No evidence of diffuse peritonitis, free fluid, or appendicolith was detected.
After a comprehensive clinical evaluation including a full history, physical examination, and review of laboratory and imaging results, the patient was consented for laparoscopic appendectomy, which was performed on the day of presentation. Intraoperatively, the appendix was severely inflamed and adherent to the small bowel, causing early mass formation with minimal turbid fluid (Figure 1). An appendectomy was performed, and a pelvic drain was inserted. On postoperative day 3, the patient was doing well, ambulating, eating, and having bowel movement. The drain was removed and the patient was discharged in good health.
A histopathological examination revealed the tip of the appendix with a pseudo-diverticulum showing acute diverticulitis along with acute suppurative appendicitis and peri-appendicitis, highly suggestive of a perforated appendix (Figure 2).
Discussion
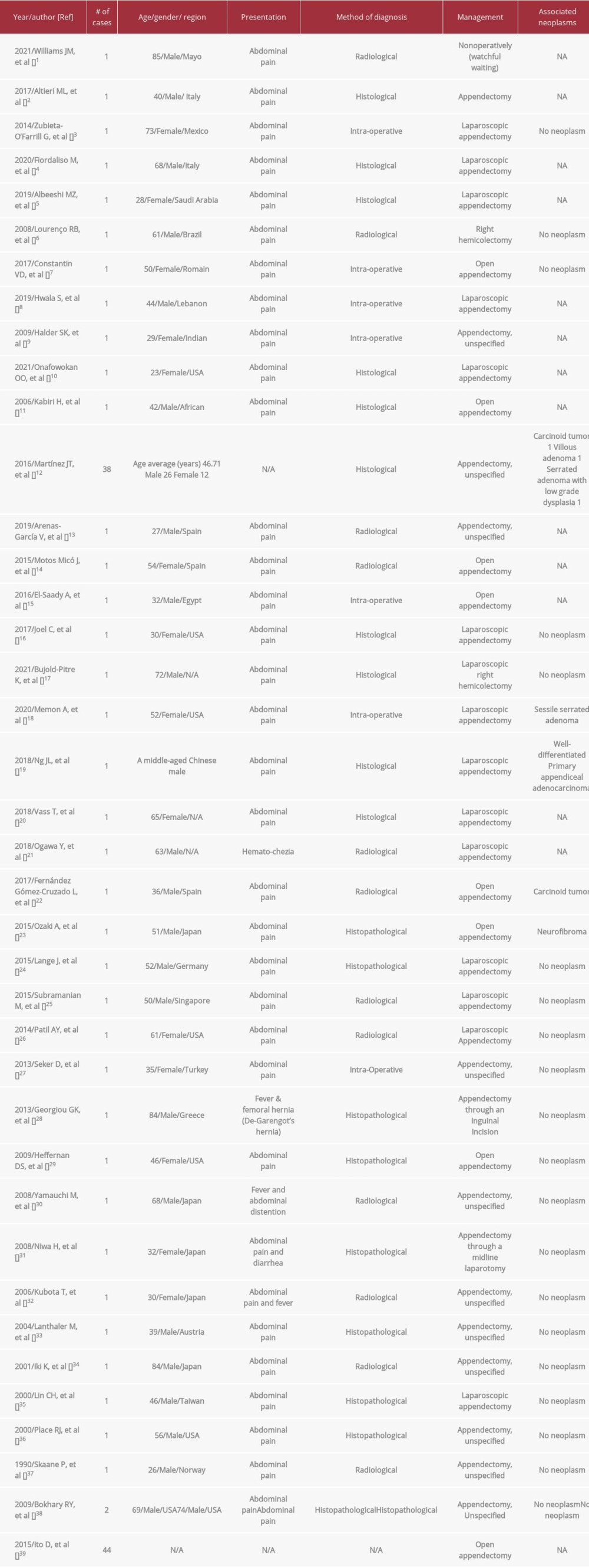
Appendiceal diverticulitis is an uncommon condition characterized by acute inflammation of a diverticulum arising from the vermiform appendix [1,2]. Appendiceal diverticulitis is usually overlooked because of its rarity and resemblance to other ileocecal diseases such as cecal diverticulitis and acute appendicitis [1,2]. Appendiceal diverticulitis accounts for 0.004–2.1% of all cases of presumed appendicitis [1,3,4]. We performed an extensive review of the English literature using the search terms “Appendiceal diverticulitis” and “Appendiceal diverticulosis” in the title, abstract, and/or keywords of articles indexed in PubMed, which is summarized in Table 1. Worldwide, there are few published cases of appendiceal diverticulitis in the literature, with only 1 case report published in our region, a 28-year-old woman reported in 2019 [5]. Appendiceal diverticulitis has a 3-fold higher risk of perforation compared to acute appendicitis. It is also associated with a greater risk of neoplasms, mainly mucinous adenomas and carcinoid tumors [1–3].
Diverticular disease of the appendix is classified into 2 types: congenital or acquired. Histologically, congenital appendiceal diverticulum is very rare, and a true diverticulum involves herniation of all 3 layers (mucosa, submucosa, and muscularis propria), while the acquired form is usually small (2–5 mm) and characterized by pseudo-diverticula (false) consistent with herniation of the mucosa and submucosa through a defect of the muscular layer, resulting from increased intraluminal pressure, considered to be acquired, like our case [1–3]. Moreover, a congenital diverticulum is usually single and located on the antimesenteric edge of the appendix, whereas acquired diver-ticula are often multiple and located in the distal third of the appendix on the mesenteric edge [2,4].
Phillips classified appendiceal diverticular disease into 4 morphological subtypes: type 1 is AD with normal appendix, type 2 is AD with acute appendicitis, type 3 is uncomplicated diver-ticulum with acute appendicitis, and type 4 is uncomplicated diverticulum with normal appendix [1,4,5]. According to this classification, our patient had type 2 appendiceal diverticulitis with ruptured acute appendicitis.
The average age at presentation of appendiceal diverticular disease was 33.6±8.33 years [3]. Based on its type, the average age of patients with congenital diverticulum is 31 years and the average age of those with acquired diverticulosis is 37 years [4]. The congenital form is believed to be associated with chromosomal anomalies such as Down syndrome (trisomy 21) and Patau syndrome (trisomy 13) [1,2]. In 1 study, trisomy 13 and 21 were reported to affect 7 of 8 patients with congenital diverticulum [4]. Other possible mechanisms of congenital diverticulum include duplication of the vermiform appendix, unsuccessful recanalization of the appendiceal lumen, adhesion traction to the appendix wall, remnant of epithelial inclusion cysts in the appendix, and failed obliteration of the vitelline duct [4].
The etiology and pathogenesis of the acquired form are unknown. One theory is that acquired appendiceal diverticula arise from a true appendiceal diverticulum, which can be either inflammatory or noninflammatory. Repeated episodes of inflammation/infection result in lymphoid tissue atrophy, which leads to a thinner residual wall and herniation. In non-inflammatory causes, increased intraluminal pressure leads to false appendiceal diverticula [4]. Risk factors related to the acquired form include male sex, diagnosis of cystic fibrosis or Hirschsprung disease, and age >30 years [2,4,5]. The incidence of acquired appendiceal diverticulosis increases to 14% in adult patients diagnosed with cystic fibrosis [4]. Our patient had some of these factors, as he was a 35-year-old man, but he did not previously report similar episodes of his condition.
Appendiceal diverticulitis (AD) is difficult to diagnose preoperatively, as it mimics acute appendicitis in clinical manifestations. For example, lower-quadrant abdominal pain is a key feature of both AD and acute appendicitis, but some differences exist; for example, it has been reported that the pain associated with AD is milder and longer lasting than that associated with appendicitis, and AD tends to affect adults aged >30 years with a history of similar episodes [4,6], whereas appendicitis is more common in children. Most patients seek medical advice once the symptoms become more prominent, and the risk of perforation increases [6]. Generally, the risk of perforation of AD has been shown to be 3–4 times higher than that of acute appendicitis, and this is associated with a 30-fold higher mortality risk [1,3,7]. In our case, the duration of symptoms was only 1 day, but presented with features of perforation on the radiological findings. The risk of perforation in acquired diverticulosis is higher than that of congenital diverticulosis (up to 66% vs 6.6%, respectively) due to a thin wall and lack of a thick muscularis propria [4,6].
Pathological evaluation of the resected specimen is required for the definitive diagnosis of appendiceal diverticulitis. Preoperative imaging using ultrasonography (US) and CT scans can be helpful to aid diagnosis, but their utility is highly radiologist-dependent. US findings of AD include a focal or diffusely thickened appendiceal wall (>3 mm), non-compressible firm dilated organ, and well-defined hypoechoic round or oval-shaped cysts attached to the enlarged appendix. The diverticulum neck may be identified at the level of the vascular hiatus under optimal conditions with adjacent inflammatory changes [6,8]. In the case of perforation, the diverticulum wall will appear in discontinuity with presence of fluid collection [6].
The use of CT to detect AD is controversial, as some studies reported that CT scans can overlook the diverticulum because of its small size and cannot differentiate AD from cecal diverticulitis or acute appendicitis [4,6]. However, another study compared the specificity and sensitivity of CT scans to US in detecting AD, and reported rates of 100% and 80%, respectively, and more than 95% in the diagnosis of acute appendicitis [3,4,6].
CT findings of AD include appendicular thickening, absence of an appendicolith or fluid collection in the appendix, and the formation of phlegmon or an abscess [4,8].
Appendectomy is the standard management of AD, either open or laparoscopic, performed safely and without any delay to prevent rupture of the appendix and subsequent risk of peritoneal seeding and pseudomyxoma peritonei if associated with an appendiceal neoplasm [2,4,8].
Conclusions
Appendiceal diverticulitis is a rare surgical entity and is usually an incidental finding on histopathological examination. It can mimic acute appendicitis in its clinical presentation, but it is associated with a higher rate of complications such as perforation and an increased risk of appendiceal neoplasms. Appendectomy is a safe and appropriate treatment for appendiceal diverticulitis.
Figures
References:
1.. Williams JM, Adamo DA, Olson MC, Acute appendiceal diverticulitis: A case report: Radiol Case Rep, 2021; 16; 1072-74
2.. Altieri ML, Piozzi GN, Salvatori P, Appendiceal diverticulitis, a rare relevant pathology: Presentation of a case report and review of the literature: Int J Surg Case Rep, 2017; 33; 31-34
3.. Zubieta-O’Farrill G, Guerra-Mora JR, Gudiño-Chávez A, Appendiceal diverticulum associated with chronic appendicitis: Int J Surg Case Rep, 2014; 5; 961-63
4.. Fiordaliso M, De Marco AF, Costantini R, A case of Type 2 appendiceal diverticulum perforated and a review of the literature: Int J Surg Case Rep, 2020; 77; 450-53
5.. Albeeshi MZ, Alwanyan AA, Salim AA, Albabtain IT, Appendiceal diverticulitis presenting as acute appendicitis diagnosed postoperatively: J Surg Case Rep, 2019; 2019; rjz332
6.. Lourenço RB, Pinho Mda C, Schraibman V, Perforated diverticulitis of the appendix: Ultrasonographic diagnosis: Einstein (Sao Paulo), 2011; 9; 75-77
7.. Constantin VD, Carâp A, Nica A, Appendiceal diverticulitis – a case report: Chirurgia (Bucur), 2017; 112; 82-85
8.. Hwala S, Aoun C, El Hajj I, Appendiceal diverticulitis presenting with clinical features of acute appendicitis: Case report and literature review: World J Surg Surgical Res, 2019; 2; 1137
9.. Halder SK, Khan I, An Indian female presenting with appendicular diverticulitis: A case report and review of the literature: Cases J, 2009; 2; 8074
10.. Onafowokan OO, Khairat A, Bonatti HJR, Appendiceal diverticulitis in a young female diagnosed on pathology after laparoscopic appendectomy for acute appendicitis: Case Rep Med, 2021; 2021; 2508956
11.. Kabiri H, Clarke LE, Tzarnas CD, Appendiceal diverticulitis: Am Surg, 2006; 72(3); 221-23
12.. Martínez JT, Deniz JR, Blanc IL, Appendiceal diverticulosis in acute appendicitis: Our experience and literature review: J Gen Pract (Los Angel), 2016; 4; 279
13.. Arenas-García V, Santos-Seoane SM, Delgado-Sevillano RJ, Appendiceal diverticulitis: An uncommon cause of acute abdomen: Rev Gastroenterol Méx (Engl Ed), 2019; 84(2); 243-44
14.. Motos Micó J, Ferrer Márquez M, Berenguel Ibáñez MdM, Appendiceal diverticulitis: A possible diagnosis in acute abdomen: Cir Esp (Engl Ed), 2015; 93(6); e49-e51
15.. El-Saady A, Diverticulitis of the appendix: Is it clinically significant?: The Egyptian Journal of Surgery, 2016; 35(2); 150-53
16.. Joel C, Noubar K, Diverticulitis of the appendix: Rare but real: Ann Emerg Surg, 2017; 2(5); 1024
17.. Bujold-Pitre K, Mailloux O, Diverticulitis of the appendix – case report and literature review: J Surg Case Rep, 2021; 2021(10); rjab488
18.. Memon A, Stoeckle DB, Incidental finding of diverticulosis of the appendix with sessile serrated adenoma: Cureus, 2020; 12(5); e8230
19.. Ng JL, Wong SL, Mathew R, Appendiceal diverticulosis: A harbinger of underlying primary appendiceal adenocarcinoma?: J Gastrointest Oncol, 2018; 9(2); E1-E5
20.. Vass T, Zaránd A, Horányi D, Harsányi L, [Diverticulosis and diverticulitis of the vermiform appendix. Report of a case and review of the literature]: Orv Hetil, 2018; 159(19); 768-72 [in Hu]
21.. Ogawa Y, Asayama N, Nagata S, Acute gastrointestinal bleeding from appendiceal diverticulitis diagnosed preoperatively by combined short-interval computed tomography and colonoscopy: A case report: Dig Endosc, 2018; 30(3); 392-94
22.. Fernández Gómez-Cruzado L, Prieto Calvo M, Pérez González C, Larrea Oleaga J, Diverticulitis of the appendix as debut of appendicular cystadenoma and carcinoid tumor: Rev Esp Enferm Dig, 2017; 109(2); 145-46
23.. Ozaki A, Tsukada M, Watanabe K, Perforated appendiceal diverticulitis associated with appendiceal neurofibroma in neurofibromatosis type 1: World J Gastroenterol, 2015; 21(33); 9817-21
24.. Lange J, Bachmann R, Königsrainer A, Zdichavsky M, Appendiceal diverticulitis shortly after a performed laparoscopic sigma resection: J Surg Case Rep, 2015; 2015(7); rjv086
25.. Subramanian M, Chawla A, Chokkappan K, Liu H, Diverticulitis of the appendix, a distinctive entity: Preoperative diagnosis by computed tomography: Emerg Radiol, 2015; 22(5); 609-12
26.. Patil AY, Levine MS, Grujic E, Goren RA, Clinical and CT findings in appendiceal diverticulitis: Clin Imaging, 2014; 38(3); 350-52
27.. Seker D, Seker G, Kahramanca S, A rare but distinctive cause of acute abdomen: appendiceal diverticulitis: J Emerg Med, 2013; 44(1); e61-62
28.. Georgiou GK, Bali C, Theodorou SJ, Appendiceal diverticulitis in a fem-oral hernia causing necrotizing fasciitis of the right inguinal region: Report of a unique case: Hernia, 2013; 17(1); 125-28
29.. Heffernan DS, Saqib N, Terry M, A case of appendiceal diverticulitis, and a review of the literature: Ir J Med Sci, 2009; 178(4); 519-21
30.. Yamauchi M, Miyamoto M, Takeuchi K, Fukuhara T, Sonographic appearance of appendiceal diverticulitis: Intern Med, 2008; 47(12); 1153-54
31.. Niwa H, Hiramatsu T, A rare presentation of appendiceal diverticulitis associated with pelvic pseudocyst: World J Gastroenterol, 2008; 14(8); 1293-95
32.. Kubota T, Omori T, Yamamoto J, Sonographic findings of acute appendiceal diverticulitis: World J Gastroenterol, 2006; 12(25); 4104-5
33.. Lanthaler M, Nehoda H, It is not always appendicitis: Wien Klin Wochenschr, 2004; 116(1–2); 51-54
34.. Iki K, Echigo M, Nogami A, Preoperative diagnosis of acute appendiceal diverticulitis by ultrasonography: Surgery, 2001; 130(1); 87-89
35.. Lin CH, Chen TC, Diverticulosis of the appendix with diverticulitis: Case report: Chang Gung Med J, 2000; 23(11); 711-15
36.. Place RJ, Simmang CL, Huber PJ, Appendiceal diverticulitis: South Med J, 2000; 93(1); 76-79
37.. Skaane P, Strøm EH, Peridiverticulitis of the appendix: An unusual ultrasonic “target lesion”: J Clin Gastroenterol, 1990; 12(3); 353-56
38.. Bokhary RY, Riddell RH, Appendiceal diverticulitis and epithelialization of the serosal surface: JKAU: Med Sci, 2009; 16(4); 93-102
39.. Ito D, Miki K, Seiichiro S, Hata S, Clinical and computed tomography findings of appendiceal diverticulitis vs acute appendicitis: World J Gastroenterol, 2015; 21(13); 3921-27
Figures
In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250