10 January 2022: Articles 
Clinically Isolated Brainstem Progressive Multifocal Leukoencephalopathy: Diagnostic Challenges
Mistake in diagnosis
Faisal KhanDOI: 10.12659/AJCR.935019
Am J Case Rep 2022; 23:e935019
Abstract
BACKGROUND: Acute brainstem syndrome (ABS), as the initial manifestation of progressive multifocal leukoencephalopathy (PML), is rarely reported. Appropriate history and neurodiagnostic testing are essential to encompass the extended spectrum of clinical and radiological differentials of ABS.
CASE REPORT: A 47-year-old woman presented to the emergency department with slurred speech, dizziness, right-sided facial droop, and numbness. Brain magnetic resonance imaging (MRI) revealed non-enhancing hyperintensities in the right lateral pons and brachium pontis, eventually extending to the bilateral middle cerebellar peduncle, pons, left>right cerebellar hemisphere, right thalamocapsular region, and midbrain region. Lumbar puncture revealed 3 cerebrospinal fluid-specific oligoclonal bands. Initial diagnosis of multiple sclerosis led to high-dose intravenous steroid treatment. The patient continued to deteriorate, leading to multiple emergency department visits and hospital admissions. Additional history revealing previously diagnosed, treatment-naive HIV prompted a repeat lumbar puncture. Cerebrospinal fluid polymerase chain reaction (PCR) for JC polyomavirus (JCV) was positive, leading to the diagnosis of clinically isolated brainstem PML. Unfortunately, the patient developed pneumonia and hypoxic respiratory failure, which ultimately led to her death.
CONCLUSIONS: This case highlights the need for considering isolated brainstem PML, as a diagnostic possibility, in patients presenting with acute-subacute brainstem symptoms and compatible neuroimaging findings. Clinicians need to be aware of varying PML presentations with brainstem or diencephalic variants, as well as monofocal lesions. The prognosis for PML has improved somewhat, secondary to immune reconstitution by highly active antiretroviral therapy, risk stratification in drug-induced PML, and other emerging treatments, such as pembrolizumab.
Keywords: Brain Stem, HIV, Leukoencephalopathy, Progressive Multifocal, Brain, Female, Humans, JC Virus, Magnetic Resonance Imaging
Background
Clinically isolated brainstem progressive multifocal leukoencephalopathy, as the initial manifestation of acquired immunodeficiency syndrome (AIDS), has rarely been reported. However, when patients present with acute-subacute brainstem symptoms, physicians should consider PML as the diagnostic possibility in the appropriate clinical and radiological context. The clinical features are extremely variable and consist of a combination of bulbar and cerebellar deficits, along with cranial nerve palsies [1]. Neuroimaging findings reveal non-enhancing T2-weighted fluid attenuated inversion recovery (FLAIR) hyperintensities and T1-weighted hypointensities in the brainstem, with or without cerebellar peduncle involvement. In later stages, diencephalic and cerebellar hemispheric involvement can be seen [1].
Here we present a case of a 47-year-old woman who presented to the emergency department (ED) with slurred speech, dizziness, and right-sided facial droop and numbness. Brain MRI revealed non-enhancing hyperintensities in the pons and brachium pontis. Three American board-certified neuroradiologists and 2 neurologists were unable to attribute the clinical and MRI findings to a diagnosis of isolated brainstem PML. A thorough history and appropriate recognition of neuroimaging characteristics on the initial presentations should have resulted in an early diagnosis and initiation of highly active antiretroviral therapy (HAART).
Case Report
A 47-year-old woman with a past medical history of hypertension, hyperlipidemia, and uterine fibroids presented to the ED (Figure 1) with a 3-day history of intermittent slurred speech, dizziness, and right-sided facial droop and numbness. The patient denied headaches and fever. She reported a history of alcohol use, but denied smoking and illicit drug use. Vital signs on arrival were blood pressure 143/90 mmHg, heart rate 89 beats/minute, respiratory rate 20 breaths/minute, temperature 98.8∘F, and SpO2 99% on room air. Neurological examination showed mild dysarthria, central type right-sided facial droop, abnormal finger-to-nose/heel-to-shin on the right, and an ataxic gait, with a positive Romberg sign. She was alert and oriented to person, place, time, and situation.
Complete blood count (CBC) and comprehensive metabolic panel (CMP) were within normal limits except for hemoglobin (Hb), which was 10.5 g/dL, and white blood cell count (3.4 K/CMM). Lipid panel revealed cholesterol 231 mg/dL, triglycerides 101 mg/dL, low-density lipoprotein 176 mg/dL, and high-density lipoprotein 44 mg/dL. Urine drug screen was negative. Other laboratory tests showed hemoglobin A1C 5.5%, vitamin B12 407 pg/mL, folate 7.3 µg/L and thyroid stimulating hormone 3.60 mIU/L. Chest X-ray showed clear lungs with no evidence of consolidation or masses. Non-contrast brain computed tomography (CT) revealed mild parenchymal volume loss, with no associated edema, infarct, or hemorrhage.
The patient was admitted and subsequent brain MRI revealed non-enhancing T2-weighted hyperintensities in the right lateral pons and right brachium pontis, with no mass effect (Figure 2). Brain and neck magnetic resonance angiography showed no evidence of stenosis or aneurysm in the circle of Willis or in the carotid or vertebral arteries. Transthoracic echocardiogram showed a preserved ejection fraction with no valvular abnormality. Cerebrospinal fluid (CSF) analysis revealed a red blood cell count of 7 K/CMM, protein 55 g/dL, albumin 25.1 g/dL, glucose 40 mg/dL, IgG 14.4 (serum IgG 1,830 mg/dL), IgG index 0.71, and 3 CSF-specific oligoclonal bands. A presumptive diagnosis of multiple sclerosis was made and the patient was treated with IV methylprednisolone, 1 g daily, for 5 days. The patient was discharged to home and instructed to follow up with neurology in the clinic. One week later, she followed up with outpatient neurology and complained of continued slurred speech and right-sided facial numbness and weakness. Glatiramer acetate 40 mg/mL, 1 mL subcutaneously, 3 times per week, was initiated, along with a referral to speech therapy.
However, the patient returned to the ED 1 week later, due to worsening dysarthria and gait ataxia. The patient denied fever, headache, chest pain, or loss of consciousness. Vital signs on arrival were blood pressure 132/86 mmHg, heart rate 101 beats/minute, respiratory rate 20 breaths/minute, temperature 99.1∘F, and SpO2 98% on room air. Physical examination revealed the patient was not distressed. Cardiopulmonary examination was within normal limits except for mild tachycardia (101 beats/minute). Neurological examination showed the patient was alert and oriented, but had worsening right-sided facial weakness and dysarthria. She also had a new left-sided hemiparesis 4/5, abnormal left-sided finger-to-nose/heel-to-shin testing, gait ataxia, and a positive Romberg sign. The CBC and CMP were within normal limits except for Hb 11.8 g/dL. Brain MRI at week 3 revealed non-enhancing patchy abnormal signals in the bilateral middle cerebellar peduncles, pons, and deep left cerebellar white matter with no mass effect. Thin rims along the superior and inferior floor of the fourth ventricle were also seen (Figure 3), suggestive of neuromyelitis optica spectrum disorder (NMOSD). The patient refused admission, was discharged home on oral prednisone 60 mg for 5 days and was advised to follow up with outpatient neurology.
One week later, she reported mild improvement, but still had significant residual deficits. Aquaporin-4 IgG antibody was found to be negative. At this time, the patient was switched from glatiramer acetate to teriflunomide 14 mg orally, once daily, due to needle phobia. Discussion for referral to a tertiary care neuroimmunology clinic was initiated; however, the patient refused.
Three weeks later, the patient’s daughter brought the patient to the ED again, with worsening neurological symptoms. Vital signs revealed blood pressure 152/79 mmHg, heart rate 118 beats/minute, respiratory rate 18 breaths/minute, temperature 101.3°F (39.8°C), and SpO2 99% on room air. The CBC and CMP were within normal limits. Testing for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) PCR was negative. Cardiopulmonary examination was within normal limits except for tachycardia (118 beats/minute). Neurological examination revealed she was lethargic, with a limited attention span, oriented to person and place, and had right-gaze palsy, worsening right-sided facial paralysis, severe dysarthria with incomprehensible speech, and hemiplegia of the left upper and lower extremities (0/5). Gross cortical and peripheral sensations were intact. Coordination testing showed abnormal finger-to-nose/heel-to-shin testing on the right side and inability to perform on the left, due to hemiplegia. Gait testing was not performed as she was unable to get out of bed. Electrocardiogram revealed sinus tachycardia and right axis deviation. Chest X-ray showed that the lungs were clear with no evidence of consolidation, pleural effusion, pneumothorax, or masses. Non-contrast head CT revealed prominent hypoattenuation of the brainstem, extending to bilateral middle cerebellar peduncles, primarily on the left side. She was started on high-dose (1 g) daily IV methylprednisolone.
The patient was admitted (Figure 1) with brain MRI, at week 7, revealing confluent areas of abnormal T2-weighted signals in the bilateral middle cerebellar peduncle, pons, left>right cerebellar hemisphere, and right thalamocapsular and midbrain regions (Figure 4A) with corresponding T1-weighted hypointensities (Figure 4B). After 5 days of treatment, the patient continued to clinically deteriorate. On further questioning, the patient hesitantly admitted a history of treatment-naive HIV, which was confirmed with a positive multispot HIV-1/HIV-2 rapid test, and CD4+ count of 40 cells/µL. Lumbar puncture revealed red blood cell count 12/CMM, white blood cell count 4/CMM, and protein 40 g/dL. Cerebrospinal fluid analysis was positive for JC polyomavirus (JCV) via PCR testing, showing 46,400 copies/mL, but was negative for herpes simplex virus I/II, cytomegalovirus, varicella zoster virus, and
Antinuclear antibody testing was also negative. On day 6 of admission, the patient developed severe shortness of breath; thus, non-invasive ventilation with bi-level positive airway pressure was initiated. A diagnosis of aspiration pneumonia versus
Discussion
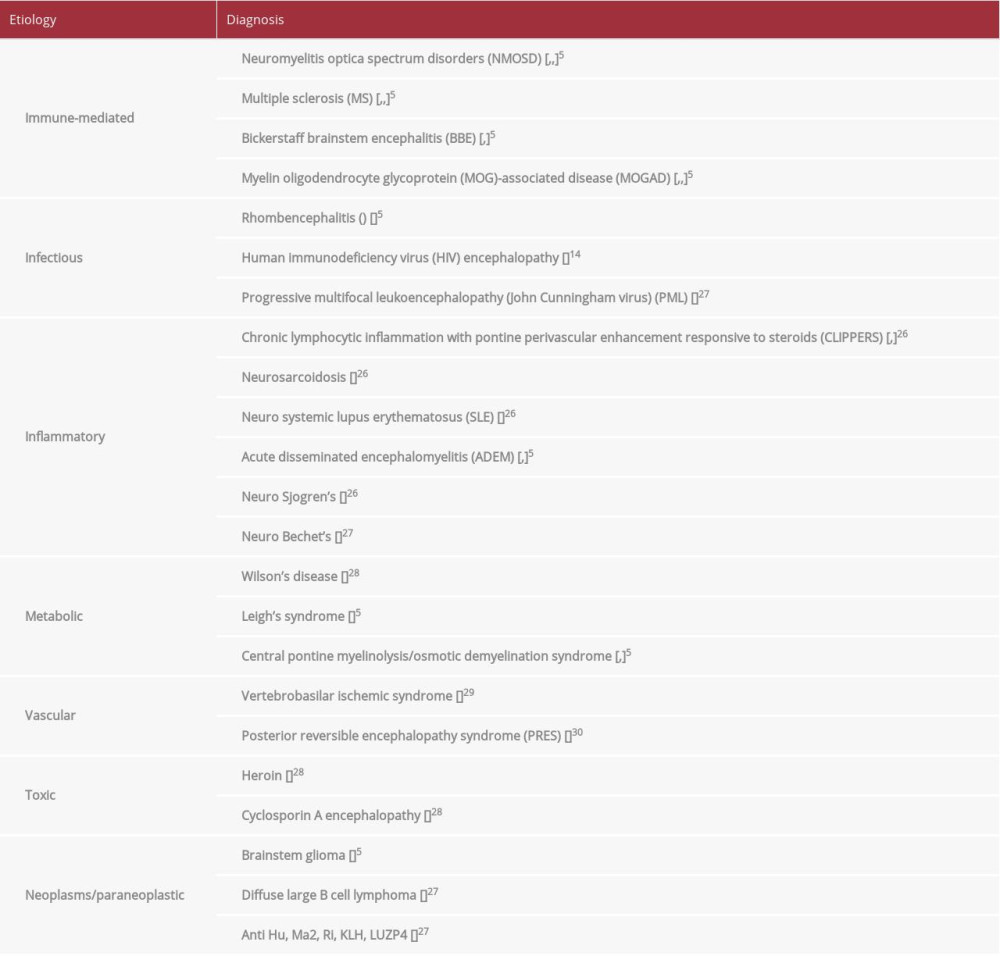
Acute brainstem syndrome presents a difficult diagnostic challenge, due to varying symptomatology and a multitude of etiologies; thus, a broad differential needs to be considered (Table 1). Our patient presented clinical and radiological findings consistent with ABS. However, diagnosis remained a dilemma, despite extensive neurodiagnostic testing.
Brainstem multiple sclerosis was the first differential considered in our patient, based on the clinical, neuroradiological, and CSF findings. Brainstem involvement in MS occurs in 29–58% of patients, with the brainstem being the initial site of lesions in up to 20% of patients [2,3]. Clinical features of MS include diplopia (68%), facial sensory problems (32%), gait imbalances (30.7%), vertigo (18.7%), oscillopsia (14.7%), facial weakness and hemispasm (14.7%), nausea and/or vomiting (13.3%), trigeminal neuralgia (13.3%), and dysarthria (constant/paroxysmal) (9.3%), among others [2,3]. Magnetic resonance imaging findings suggestive of brainstem MS include well-defined, solitary, or confluent lesions in the floor of the fourth ventricle, middle cerebellar peduncles (49–88%), brainstem periphery, or, especially, the pons, and can be contiguous with cerebrospinal fluid cisterns [4–6]. Our patient fulfilled the 2017 McDonald criteria for MS, based on clinical, radiological, and CSF findings [7]. However, due to her rapid and persistent clinical and radiological progression, other differentials were considered.
Acute brainstem syndrome, a subset of NMOSD, was also considered. The clinical presentation is highly variable and consists of diplopia, dysarthria, dysphagia, facial paralysis, ataxia, intractable nausea, vomiting, and hiccups [8]. Previously described neuroimaging findings include lesions in the pons and medulla, adjacent to the fourth ventricle, including the area postrema, that often extend to the cervical cord [8,9]. Our patient presented with facial paralysis and gait ataxia, and had brain MRI findings of a thin rim along the superior and inferior floor of the fourth ventricle, with patchy hyperintensities in the bilateral middle cerebellar peduncles, pons, and deep left cerebellar white matter. However, the AQP-4 IgG antibody test was negative, ruling out seropositive NMOSD. Additionally, our patient did not have a history of optic neuritis, transverse myelitis, or area postrema syndrome, ruling out the possibility of seronegative NMOSD [10].
We also contemplated neurosarcoidosis (NS) as a differential based on our patient’s age and ethnicity (African-American). However, she did not have any signs or symptoms associated with systemic sarcoidosis. Additionally, the lack of parenchymal or leptomeningeal contrast enhancement on neuro-imaging, and poor response to steroid treatment negated the possibility of neurosarcoidosis [11]. Another differential considered was chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). However, the lack of response to steroids ruled out CLIPPERS as a diagnostic possibility.
Our patient’s final diagnosis was clinically isolated brainstem progressive multifocal leukoencephalopathy (PML). This is an extremely rare pathology, with only 10 reported cases, as summarized by Breville et al [1]. Cerebral PML with secondary brainstem involvement is more commonly reported than isolated brainstem PML. To the best of our knowledge, we are reporting the eleventh case of clinically isolated brainstem PML, which later extended into the diencephalon. The demographics of isolated brainstem PML seems to be synonymous with supratentorial PML. In immunocompromised JCV-seropositive individuals, PML has been found to be more common in the setting of AIDS (55–85%) [6]. Breville et al found that 7 of the 10 patients who were reported to have isolated brainstem PML were HIV positive [1].
Clinical features of isolated brainstem PML include unilateral facial weakness, dysphagia, dysarthria, diplopia, nystagmus, unilateral arm intention tremor, gait ataxia, titubation, and dysdiadochokinesia [1]. This is strikingly different from cerebral PML presentation, where gait ataxia (65%), cortical hemiparesis (60%), cognitive impairment and personality changes (30%), visual impairment (20%), and seizures (10%) dominate the clinical picture [12,13]. Our patient’s clinical presentation was consistent with acute brainstem syndrome, including intermittent slurred speech, right-sided facial droop and numbness, and right-gaze palsy.
Brain MRI features seen in all reported cases of isolated brainstem PML are non-enhancing T2-weighted FLAIR hyperintensities and T1-weighted hypointensities without mass effect [1]. These lesions are patchy and progressively worsen over time [1], similar to our case. Since most cases of isolated brainstem PML, in the setting of HIV, were discovered during the late stages of the disease, little is known about MRI features in the early stages of HIV-induced isolated brainstem PML [14]. In our patient, her initial brain MRI revealed non-enhancing T2-weighted hyper-intensities in the right lateral pons and brachium pontis. These lesions progressively worsened and extended to involve the bilateral middle cerebellar peduncles, thalamus, midbrain, deep left cerebellar white matter, and floor of the fourth ventricle. In contrast, the neuroimaging features of cerebral PML are characterized by lesions involving the corpus callosum and parieto-occipital regions [15,16]. They are multifocal, asymmetric, bilateral, non-enhancing, and found not only in the subcortical white matter (especially U fibers), but also in the deep gray matter, juxtacortical regions, and the cortex [15]. MRI findings typically reveal hyperintensities on T2-weighted FLAIR images and iso-/ hypointensities on T1-weighted images, without mass effect or edema, a classic “milky-way” pattern, and barbell sign [15,16]. In previous cases, apparent diffusion coefficient and diffusion weighted imaging (DWI) MRI sequences have revealed patchy, peripheral diffusion restriction at the leading edge of the lesions [17]. However, in the present case, 3 board-certified neuroradiologists and 2 neurologists, from different facilities, were unable to identify these lesions as isolated brainstem PML.
Brain biopsy has a sensitivity of 93–96%. However, it is not feasible in brainstem pathologies, so the clinician needs to rely on ancillary diagnostic modalities [18]. Moreover, the need for a brain biopsy for the diagnosis of PML has been obviated, secondary to the development of ultrasensitive (>95%) CSF PCR, to detect JCV DNA [19,20]. JCV undergoes mutations of the VP1 gene, causing the virus to become neurotropic, thus leading to invasion of oligodendrocytes and potentially astrocytes [15]. The VP1 gene mutation has been suggested to pre-dispose susceptible patients to an aggressive PML course [15]. Our diagnosis of brainstem PML was made based on clinical, radiological, and CSF findings. The VP1 gene analysis was not available, as this was not part of the pathology lab protocol.
Clinicians and radiologists should be aware of varying presentations and imaging characteristics in the evolving landscape of PML. An increased incidence of PML has been seen due to recent advancements in the treatment of inflammatory or immunological conditions including MS, psoriasis, and rheumatoid arthritis. Various immunosuppressants such as steroids, methotrexate, cyclophosphamide, cyclosporine, rituximab, vincristine, and natalizumab have been reported to induce PML [13,21,22]. Natalizumab, one of the most commonly reported culprits among drug-induced PML, results in monofocal, uni-lateral, and frontal lobe lesions, as compared with classic cerebral PML, which is characterized by lesions that are multifocal, asymmetric, bilateral, and parieto-occipital in location [15]. In the Breville et al literature review, 2 out of 10 isolated brainstem PML cases were natalizumab induced [1].
Unfortunately, there is no definitive treatment for PML, and managing the underlying immunosuppression is the best option. Mortality rates in AIDS-induced PML are reduced from 90% to 50% with the use of HAART [23]. The median survival of HAART-naive PML is 6 months, with only 9% living more than 1 year after initial diagnosis; this increases to 50% with the use of HAART [13,15]. Cortese et al found that the use of pembrolizumab, a monoclonal antibody targeting programmed cell death 1 (PD-1) inhibitors, reduces JCV viral load and thereby prevents the recurrence of PML [24,25]. However, large scale clinical trials regarding the use of pembrolizumab are still ongoing. Other proposed treatment options include mirtazapine, cidofovir, and allogeneic BK virus–specific T cells [16,22].
Immune reconstitution inflammatory syndrome-PML (IRIS-PML), is an unfortunate complication of HAART, in the setting of HIV and PML. The mortality rate is high (5–28%), and occurs in 4–42% of HIV patients with superimposed JCV infection [15]. Brain MRI shows contrast enhancement within PML lesions (56–87%), vasogenic edema (30%), and mass effect (24%) [15]; these features are characteristically absent in cerebral and brainstem PML [15]. Our patient never started HAART; thus, IRIS-PML was an unlikely contributor to our patient’s demise.
In PML patients, both primary and secondary brainstem involvement carries a poor prognosis [1]. Other poor prognostic factors include CD4+ count < 200 cells/µL, high JCV CSF load, and delayed diagnosis and management [1]. Our patient fulfilled all of these criteria, hence contributing to her unfortunate death.
Conclusions
Isolated brainstem PML needs to be considered in patients presenting with acute-subacute brainstem symptoms, along with compatible neuroimaging findings. Symptomatology is highly variable, thus requiring a high index of clinical suspicion. The PML presentation may also continue to evolve, secondary to the advent of newer risk factors, especially immunosuppressants. Although cerebral PML remains the predominant form, brainstem and diencephalic variants are being increasingly recognized. Neuroradiological findings of brainstem PML follow the imaging characteristics of cerebral PML with non-enhancing, T2-weighted FLAIR hyperintensities and T1-weighted hypointensities, without mass effect. The development of ultrasensitive CSF PCR for JCV has improved diagnostic accuracy and has obviated the need for invasive procedures, such as brain biopsy. The prognosis has improved somewhat, secondary to early initiation of HAART, risk stratification prior to initiation of immunosuppressants, neuro-imaging surveillance, and other emerging treatments.
Figures
References:
1.. Breville G, Koralnik IJ, Lalive PH, Brainstem progressive multifocal leukoencephalopathy: Eur J Neurol, 2021; 28(3); 1016-21
2.. Lu Z, Zhang B, Qiu W, Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis: PLoS One, 2011; 6(8); e22766
3.. Habek M, Evaluation of brainstem involvement in multiple sclerosis: Expert Rev Neurother, 2013; 13(3); 299-311
4.. Morales H, Tomsick T, Middle cerebellar peduncles: Magnetic resonance imaging and pathophysiologic correlate: World J Radiol, 2015; 7(12); 438-47
5.. Quattrocchi CC, Errante Y, Rossi Espagnet MC, Magnetic resonance imaging differential diagnosis of brainstem lesions in children: World J Radiol, 2016; 8(1); 1-20
6.. Barkhof F, Koeller KK, Demyelinating diseases of the CNS (brain and spine): Diseases of the brain, head and neck, spine 2020–2023: Diagnostic imaging [Internet] Feb 15, 2020, Cham (CH), Springer 2020; Chapter 13
7.. Thompson AJ, Banwell BL, Barkhof F, Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria: Lancet Neurol, 2018; 17(2); 162-73
8.. Cheng C, Jiang Y, Lu X, The role of anti-aquaporin 4 antibody in the conversion of acute brainstem syndrome to neuromyelitis optica: BMC Neurol, 2016; 16(1); 203
9.. Kim HJ, Paul F, Lana-Peixoto MA, Guthy-Jackson Charitable Foundation NMO International Clinical Consortium & Biorepository. MRI characteristics of neuromyelitis optica spectrum disorder: An international update: Neurology, 2015; 84(11); 1165-73
10.. Wingerchuk DM, Banwell B, Bennett JL, International Panel for NMO Diagnosis. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders: Neurology, 2015; 85(2); 177-89
11.. Pirau L, Lui F, Neurosarcoidosis: StatPearls [Internet] Jul 6, 2021; 2021, Treasure Island (FL), StatPearls Publishing
12.. Williamson EML, Berger JR, Diagnosis and treatment of progressive multifocal leukoencephalopathy associated with multiple sclerosis therapies: Neurotherapeutics, 2017; 14(4); 961-73
13.. Aksamit AJ, Progressive multifocal leukoencephalopathy: Continuum (Minneap Minn), 2012; 18(6 Infectious Disease); 1374-91
14.. Gonzalez Caldito N, Loeb JS, Okuda DT, Neuroimaging insights into early stages of HIV-progressive multifocal leukoencephalopathy: A case report: J Cent Nerv Syst Dis, 2020; 12; 1179573520939339
15.. Cortese I, Reich DS, Nath A, Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease: Nat Rev Neurol, 2021; 17(1); 37-51
16.. Grill M, Neurologic complications of human immunodeficiency virus: Continuum (Mineap Minn), 2021; 27(4); 963-91
17.. Buckle C, Castillo M, Use of diffusion-weighted imaging to evaluate the initial response of progressive multifocal leukoencephalopathy to highly active antiretroviral therapy: Early experience: Am J Neuroradiol, 2010; 31(6); 1031-35
18.. Berger JR, Aksamit AJ, Clifford DB, PML diagnostic criteria: Consensus statement from the AAN Neuroinfectious Disease Section: Neurology, 2013; 80(15); 1430-38
19.. Nakamichi K, Kawamoto M, Ishii J, Saijo M, Improving detection of JC virus by ultrafiltration of cerebrospinal fluid before polymerase chain reaction for the diagnosis of progressive multifocal leukoencephalopathy: BMC Neurol, 2019; 19(1); 252
20.. Major EO, Yousry TA, Clifford DB, Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: A decade of lessons learned: Lancet Neurol, 2018; 17(5); 467-80
21.. Yukitake M, Drug-induced progressive multifocal leukoencephalopathy in multiple sclerosis: A comprehensive review: Clin Exp Neuroimmunol, 2018; 9; 37-47
22.. Bennett CL, Focosi D, Socal MP, Southern Network on Adverse Reactions. Progressive multifocal leukoencephalopathy in patients treated with rituximab: A 20-year review from the Southern Network on Adverse Reactions: Lancet Haematol, 2021; 8(8); e593-e604
23.. AlTahan AM, Berger T, AlOrainy IA, AlTahan H, Progressive multifocal leukoencephalopathy in the absence of typical radiological changes: Can we make a diagnosis?: Am J Case Rep, 2019; 20; 101-5
24.. Cortese I, Muranski P, Enose-Akahata Y, Pembrolizumab treatment for progressive multifocal leukoencephalopathy: N Engl J Med, 2019; 380(17); 1597-605
25.. Pawlitzki M, Schneider-Hohendorf T, Rolfes L, Ineffective treatment of PML with pembrolizumab: Exhausted memory T-cell subsets as a clue?: Neurol Neuroimmunol Neuroinflamm, 2019; 6(6); e627
26.. Law LY, Riminton DS, Nguyen M, The spectrum of immune-mediated and inflammatory lesions of the brainstem: Clues to diagnosis: Neurology, 2019; 93(9); 390-405
27.. Zoghaib R, Sreij A, Maalouf N, Autoimmune brainstem encephalitis: An illustrative case and a review of the literature: J Clin Med, 2021; 10(13); 2970
28.. de Oliveira AM, Paulino MV, Vieira APF, Imaging patterns of toxic and metabolic brain disorders: Radiographics, 2019; 39(6); 1672-95
29.. Miller DH, Weinshenker BG, Filippi M, Differential diagnosis of suspected multiple sclerosis: a consensus approach: Mult Scler, 2008; 14(9); 1157-74
30.. Hinduja A, Posterior reversible encephalopathy syndrome: Clinical features and outcome: Front Neurol, 2020; 11; 71
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250