13 September 2020: Clinical Research
Celastrol Inhibits Migration and Invasion of Triple-Negative Breast Cancer Cells by Suppressing Interleukin-6 via Downregulating Nuclear Factor-κB (NF-κB)
Fei Yan1BCDE, Zihong Wu1BCD, Zihui Li1ABCD, Li Liu1BCDE*DOI: 10.12659/MSM.922814
Med Sci Monit 2020; 26:e922814
Abstract
BACKGROUND: Celastrol is extracted from the root of the Chinese traditional herb Tripterygium wilfordii, which has anti-cancer effects in multiple cancers. However, the effect of celastrol on the metastasis of triple-negative breast cancer and its mechanism remain largely unknown.
MATERIAL AND METHODS: MDA-MB-468 and MDA-MB-231 cells were treated with various doses of celastrol for 24 h. Cell viability was measured via MTT analysis. Cell migration and invasion were detected via transwell analysis. The expression of interleukin-6 (IL-6) was measured after transfection of short-hairpin RNA against IL-6 or celastrol treatment via quantitative real-time polymerase chain reaction, Western blot, or enzyme-linked immunosorbent analysis (ELISA). The protein levels in the nuclear factor-κB (NF-κB) pathway were measured by Western blot. The interaction between celastrol and NF-κB-mediated IL-6 was investigated by luciferase reporter assay.
RESULTS: High concentrations of celastrol inhibited viability of MDA-MB-468 and MDA-MB-231 cells, but low doses of celastrol showed little effect on cell viability. Low doses of celastrol suppressed cell migration and invasion, and knockdown of IL-6 also repressed cell migration and invasion. Moreover, treatment with celastrol decreased IL-6 expression at mRNA and protein levels. IL-6 overexpression mitigated celastrol-mediated suppression of cell migration and invasion. Additionally, celastrol blocked the NF-κB pathway to inhibit IL-6 levels.
CONCLUSIONS: Celastrol repressed migration and invasion through decreasing IL-6 levels by inactivation of NF-κB signaling in triple-negative breast cancer cells, providing a novel basis for use of celastrol in treating triple-negative breast cancer.
Keywords: Triple Negative Breast Neoplasms, Down-Regulation, Interleukin-6, Pentacyclic Triterpenes
Background
Breast cancer is a common cancer in women worldwide, with high incidence and mortality rates [1]. Triple-negative breast cancer (TNBC) accounts for 15–20% of all breast tumors and is characterized by frequent metastasis [2]. The metastasis involves dissemination of cancer cells and is the leading cause of mortality in breast cancer patients [3]. With advances in understanding the pathogenesis of breast cancer, many natural phytochemicals have been reported to be promising for treatment of female breast cancer [4]. However, more studies are needed to better understand their pharmacology in treating TNBC.
Celastrol, a natural phytochemical from the root of the Chinese traditional herb
Interleukin-6 (IL-6) is a multifunctional cytokine that regulates cell growth, angiogenesis, and other progressions in various cancers [10]. A previous study suggested that IL-6 may serve as a negative prognosticator in breast cancer [11]. Furthermore, IL-6 was found to be highly expressed and important for growth of TNBC cells [12]. Therefore, novel strategies directed towards Il-6 may be alternative ways to improve the outcome of TNBC. Nuclear factor-κB (NF-κB) can regulate IL-6 expression via binding sites within the IL-6 promoter area, and this regulatory network plays essential roles in breast cancer progression [13,14]. Hence, we hypothesized that NF-κB-mediated IL-6 is required in the anti-cancer effect of celastrol in TNBC. Cell migration and invasion are key steps in cancer metastasis. In this research, we assessed the function of celastrol in migration and invasion of TNBC cells and explored the potential interaction between celastrol and IL-6.
Material and Methods
CELL CULTURE AND CELASTROL EXPOSURE:
Human TNBC cell lines MDA-MB-468 and MDA-MB-231 were purchased from BeNa Culture Collection (Beijing, China). The cells were grown in DMEM (Sigma, St. Louis, MO, USA) plus 10% fetal bovine serum (Sigma) and 1% penicillin/streptomycin (Sigma) at 37°C with 5% CO2. The purity of celastrol (Sigma) was ≥98% and it was dissolved in DMSO (Sigma). Cells were incubated with various doses of celastrol (0.1, 0.5, 1, 2, and 5 μM) for 24 h based on methods described in previous reports [6,15]. The high doses (2 and 5 μM) and low doses (0.1, 0.5, and 1 μM) of celastrol were differentiated according to the influence on cell viability. The control group was treated with an equal volume of DMSO. Subsequently, cells were collected for further experiments. Each sample was prepared in triplicate, and every experiment was repeated 3 times.
CELL VIABILITY:
MDA-MB-468 and MDA-MB-231 cells (1×104/well) were added into 96-well plates overnight and then stimulated with various concentrations (0.1, 0.5, 1, 2, and 5 μM) of celastrol. After incubation for 24 h, cells were exposed to 0.5 mg/mL MTT solution (Sigma) for another 4 h. Subsequently, the medium was exposed to DMSO to solubilize the formazan. The absorbance at 490 nm was detected using a microplate reader (Bio-Rad, Hercules, CA, USA).
TRANSWELL ASSAY:
Cell migration and invasion were detected in 24-well transwell chambers. For cell migration analysis, MDA-MB-468 and MDA-MB-231 cells (3×104 cells) in 100 μL non-serum DMEM were placed in the upper chambers, and 500 μL medium with 10% fetal bovine serum was placed in the lower chambers. After incubation with various concentrations (0.1, 0.5, and 1 μM) of celastrol for 24 h, cells that migrated to the bottom of chambers were dyed with 0.5% crystal violet (Beyotime, Shanghai, China) and observed under a microscope (Nikon, Tokyo, Japan) with 3 randomly selected fields. For invasion analysis, the transwell chambers were pre-coated with 30 μg per well of Matrigel (Solarbio, Beijing, China) and the experiment was conducted the same as above.
CELL TRANSFECTION:
The overexpression vector of IL-6 (oe-IL-6) was constructed by cloning the full-length sequence of IL-6 into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA), with pcDNA3.1 as negative control (vector). Short-hairpin RNA (shRNA) against IL-6 (sh-IL-6-1 or sh-IL-6-2) and shRNA negative control (sh-NC) were obtained from Fulengen (Guangzhou, China). Cell transfection was carried out with Lipofectamine 3000 (Invitrogen). After 24 h, cells were collected for assessment of transfection efficacy and transwell analyses.
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION (QRT-PCR):
After the transfection or celastrol treatment, MDA-MB-468 and MDA-MB-231 cells were interacted with TRIzol reagent (Invitrogen) for RNA isolation. All-in-One mRNA First Strand cDNA Synthesis kits and qRT-PCR Detection kits (Fulengen) were used for RNA reverse transcription and qRT-PCR, respectively. The amplified reaction was conducted under the following conditions: 95°C for 5 min, followed by 35 cycles of 95°C for 15 s and 60°C for 1 min. The relative expression of IL-6 was analyzed with GAPDH as a reference via 2−ΔΔCt method [16].
The primer sequences were:
WESTERN BLOT ANALYSIS:
Following transfection or celastrol treatment, MDA-MB-468 and MDA-MB-231 cells were harvested and lysed in RIPA containing phenylmethanesulfonyl fluoride (Beyotime). After quantification using a BCA kit (Beyotime), protein was denatured by boiling water bath for 5 min. Then, we separated 20 μg protein samples via SDS-PAGE, transferred them to nitrocellulose membranes (Bio-Rad), and blocked them in 5% skim milk for 1 h. Subsequently, the membranes were interacted with rabbit primary antibodies overnight at 4°C, including IL-6 (#12153, Cell Signaling Technology, Danvers, MA, USA), p-IKBα (#2859), IKBα (#4812), p65 (#8242), GAPDH (#2118), and Lamin B (#13435), and then interacted with anti-rabbit horseradish peroxidase-conjugated IgG (#5127) for 2 h. The protein signaling was visualized through ECL substrate (Beyotime) and quantitated via Image Lab software (Bio-Rad). Relative protein expression was analyzed with GAPDH or Lamin B as the internal control and the normalized control, respectively.
ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA):
MDA-MB-468 and MDA-MB-231 cells (1×105/well) were added into 12-well plates and then exposed to celastrol for 24 h. The culture medium was harvested for assessment of IL-6 secretion level using a human ELISA kit (Invitrogen) following the instructions of the manufacturer. Intensity of color was detected with a microplate reader at 450 nm and the secreted level of IL-6 was measured according to the representative standard curve.
LUCIFERASE REPORTER ASSAY:
The IL-6 luciferase reporter vector carrying the NF-κB response element was constructed based on the pGL3 reporter vector (Promega, Madison, WI, USA) following a previously reported method [17], and termed as pGL3-IL-6(−149/+8). The reporter vector pGL3-IL-6(−149/+8 MUT) containing the mutant NF-κB response element was obtained using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). MDA-MB-468 and MDA-MB-231 cells at 70% confluence in 24-well plates were transfected with 100 ng luciferase reporter vector and pSV-β-galactosidase (Thermo Fisher, Wilmington, DE, USA) for 24 h. Then, the transfected cells were incubated with 1 μM celastrol for 24 h. The luciferase activity and β-galactosidase activity were measured following the instructions of the manufacturer (Promega). The luciferase activity and β-galactosidase activity were monitored using a GloMax 20/20 luminometer (Promega). The β-galactosidase activity was regarded as normalization.
STATISTICAL ANALYSIS:
Data from 3 independent experiments are expressed as the means±standard deviation. GraphPad Prism 6 (GraphPad, San Diego, CA, USA) was employed for graph drawing and analysis of differences via ANOVA followed by Dunnett’s test. A
Results
CELASTROL SUPPRESSES VIABILITY OF TNBC CELLS:
To analyze the anti-cancer function of celastrol in TNBC, MDA-MB-231, and MDA-MB-468 cells, cells were stimulated with various doses (0.1, 0.5, 1, 2, and 5 μM) of celastrol. After incubation for 24 h, cell viability was measured via MTT analysis. Results showed high doses (2 and 5 μM) of celastrol led to significant loss of viability of MDA-MB-231 compared with the non-treated group (Figure 1A). Similarly, cell viability was also obviously inhibited in MDA-MB-468 cells after exposure to high doses (2 and 5 μM) of celastrol (Figure 1B). However, low doses (0.1, 0.5, and 1 μM) of celastrol had little effect on cell viability. To eliminate the effect of viability inhibition on metastasis, cells treated with low doses (0.1, 0.5, and 1 μM) of celastrol were used for subsequent experiments.
CELASTROL SUPPRESSED MIGRATION AND INVASION OF TNBC CELLS:
To explore the influence of celastrol on metastasis, MDA-MB-468 and MDA-MB-231 cells were stimulated with low concentrations (0.1, 0.5, and 1 μM) of celastrol for 24 h. Transwell migration analysis revealed that exposure to celastrol strongly suppressed cell migration in a concentration-dependent manner (Figure 2A, 2B). Cell invasive ability was analyzed by Matrigel transwell assay, showing progressive reduction of invasive ability in the 2 cell lines after stimulation wth celastrol, in a concentration-dependent manner (Figure 2C, 2D).
KNOCKDOWN OF IL-6 REPRESSED MIGRATION AND INVASION OF TNBC CELLS:
To probe the potential role of IL-6 in TNBC, MDA-MB-231, and MDA-MB-468 cells, cells were transfected with sh-IL-6-1, sh-IL-6-2, or sh-NC. After transfection for 24 h, the expression of IL-6 was measured at transcriptional and protein levels. qRT-PCR assay results showed that transfection of sh-IL-6-1 or sh-IL-6-2 effectively decreased the mRNA level of IL-6 compared with the sh-NC group (Figure 3A). Western blot analysis showed that IL-6 protein level was significantly reduced in cells transfected with sh-IL-6-1 or sh-IL-6-2 compared with that in the sh-NC group (Figure 3B). Moreover, the secreted level of IL-6 in medium was also decreased by transfection with sh-IL-6-1 or sh-IL-6-2 (Figure 3C). These findings validated the transfection efficacy of sh-IL-6-1 or sh-IL-6-2. In addition, the effect of IL-6 on metastasis of breast cancer cells was investigated by transwell assay after transfection, showing that knockdown of IL-6 notably impeded migration and invasion of MDA-MB-231 and MDA-MB-468 cells (Figure 3D–3F).
IL-6 OVEREXPRESSION REVERSED THE SUPPRESSIVE EFFECT OF CELASTROL ON MIGRATION AND INVASION OF TNBC CELLS:
To explore whether IL-6 is involved in celastrol-induced inhibition of metastasis of TNBC, the effect of celastrol on IL-6 level was investigated in MDA-MB-231 and MDA-MB-468 cells. After incubation with low concentrations (0.1, 0.5, and 1 μM) of celastrol for 24 h, the expression of IL-6 mRNA was progressively inhibited in a concentration-dependent manner in comparison to the non-treated group (Figure 4A). Furthermore, the protein level of IL-6 was significantly decreased in these 2 cell lines after exposure to various doses of celastrol (Figure 4B). Similarly, ELISA assay revealed that the level of IL-6 was also inhibited in medium following celastrol treatment, in a concentration-dependent manner (Figure 4C). Additionally, MDA-MB-231 and MDA-MB-468 cells were transfected with oe-IL-6 or vector and then exposed to 1 μM celastrol for 24 h. After the transfection of IL-6, the mRNA and protein levels of IL-6 were markedly enhanced in cells (Figure 4D–4F). Overexpression of IL-6 weakened celastrol-mediated suppression of migration and invasion in MDA-MB-231 and MDA-MB-468 cells (Figure 4G, 4H).
CELASTROL REDUCED IL-6 LEVEL BY BLOCKING THE NF-κB PATHWAY:
To investigate the effect of celastrol on the NF-κB pathway, the expressions of protein in this signaling pathway were measured via Western blot analysis. The results of Western blot analysis showed treatment of celastrol obviously inhibited the expressions of p-IKBα and p65 but increased IKBα protein level, in a concentration-dependent manner, suggesting that celastrol inhibited activation of the NF-κB pathway (Figure 5A, 5B). Moreover, we constructed the IL-6 luciferase reporter vector containing the NF-κB response element. Luciferase reporter assay showed that treatment with celastrol strongly decreased luciferase activity of pGL3-IL-6(−149/+8) cells compared with the control group, while its efficacy was reduced by the mutation of NF-κB binding sites in the pGL3-IL-6(−149/+8 MUT) group (Figure 5C, 5D).
Discussion
Chinese herbal medicine has been widely used as adjunct breast cancer therapy [18]. Celastrol is a natural phytochemical that plays an important anti-cancer role in TNBC [9]. To the best of our knowledge, this study is the first to assess the anti-metastatic role of celastrol and to determine the interaction between celastrol and IL-6 in TNBC cells.
A growing number of studies have reported the anti-metastatic roles of celastrol in many cancers, including ovarian cancer, chondrosarcoma, and osteosarcoma [19–21]. Moreover, previous studies showed that celastrol repressed phorbol 12-myristate 13-acetate-induced migration and invasion of MCF-7 breast cancer cells at various concentrations (0.1–2.5 μM) [15]. Mi et al. found MDA-MB-231 cell viability was clearly decreased by treatment with 3 or 10 μM celastrol, but not with 1 μM. High concentrations of celastrol suppressed invasion of MDA-MB-231 cells, while exposure to 1 μM celastrol for 12 h had little effect on the level of TNF-α [22]. In the present study, we also found that treatment with 2 or 5 μM celastrol for 24 h clearly decreased viability of MDA-MB-231 and MDA-MB-468 cells, while low concentrations (0.1, 0.5 and 1 μM) of celastrol did not. To eliminate the effect of viability inhibition on migration and invasion, the transwell assay was conducted in TNBC cells after treatment with low doses (0.1, 0.5, and 1 μM) of celastrol for 24 h. Results showed that celastrol treatment suppressed TNBC cell migration and invasion, showing the anti-metastatic role of celastrol in TNBC. Although it was reported that treatment with 1 μM of celastrol for 12 h did not alter the invasion of MDA-MB-231, we hypothesize this difference might be caused by the different exposure durations. However, the potential mechanism underlying the ability of celastrol to block migration and invasion of TNBC cells remains elusive. Previous works demonstrated that celastrol could decrease IL-6 expression
IL-6 was reported to be involved in synergistic paracrine signaling to promote cell migration, and its inhibition reduced the metastatic capacity of cancer cells [25]. Furthermore, downregulation of IL-6 contributed to inhibition of cell viability, colony formation, and migration in TNBC cells [26]. In the present study, to analyze the potential function of IL-6 in cell migration and invasion, we purchased commercial sh-IL-6 products and transfected them into MDA-MB-231 and MDA-MB-468 cells. After transfection, we found the level of IL-6 was effectively reduced at mRNA and protein levels. Moreover, knockdown of IL-6 suppressed cells migration and invasion, suggesting IL-6 inhibition plays an anti-metastatic role in TNBC. Meanwhile, we found that IL-6 was highly expressed in TNBC cells, and MDA-MB-231 cells exhibited higher levels of IL-6 than MDA-MB-468 cells, which is also in agreement with previous studies [12,27]. We also validated that celastrol decreased IL-6 expression, and IL-6 overexpression reversed the effect of celastrol on cell migration and invasion, showing that celastrol blocked migration and invasion of TNBC cells by decreasing IL-6 expression. However, little is known about how celastrol inhibits IL-6 levels. The available evidence indicates that NF-κB binds with IL-6 promoter, and inhibition of NF-κB/IL-6 signaling can suppress migration and angiogenesis of TNBC [14]. We found that celastrol induced accumulation of IKBα through impeding the phosphorylation of IKBα, leading to reduction of p65, revealing that celastrol inhibited NF-κB pathway activation in TNBC cells. In addition, we found that celastrol inhibited the luciferase activity of pGL3-IL-6(−149/+8), indicating that celastrol decreased IL-6 expression by blocking the NF-κB pathway in TNBC, which is also reported in prostate cancer, dorsal root ganglion, LPS-treated retinal pigment epithelial cells, cholestatic liver injury, and gamma irradiation-induced injury [28–32]. These findings show that celastrol suppresses cell migration and invasion by inhibiting IL-6 expression via the NF-κB pathway in TNBC cells. Nevertheless,
Conclusions
In conclusion, the present study demonstrated that celastrol has an anti-cancer effect in TNBC. Celastrol inhibited migration and invasion in TNBC cells at low-toxicity concentrations. Moreover, we found that the anti-metastatic role of celastrol was associated with blocking the NF-κB/IL-6 pathway. This indicates celastrol is a promising agent for treatment of TNBC by inhibiting migration and invasion.
Figures
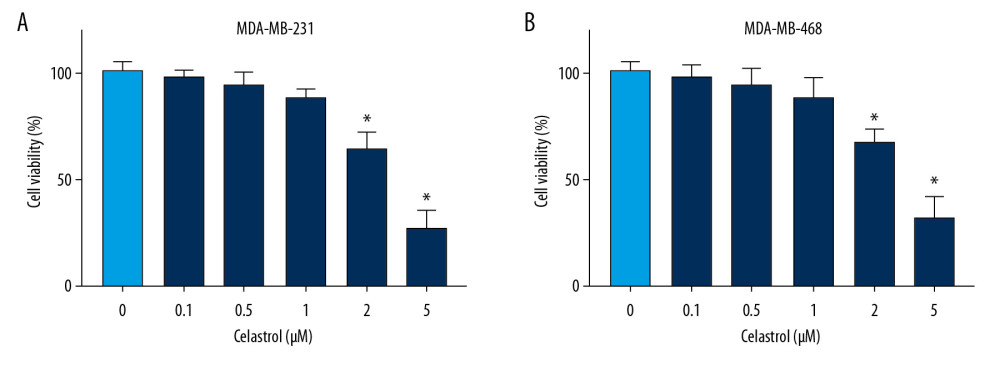 Figure 1. The effect of celastrol on viability of TNBC cells. (A, B) Cell viability was examined in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h via MTT assay. * P<0.05 vs. the non-treated group (0 μM).
Figure 1. The effect of celastrol on viability of TNBC cells. (A, B) Cell viability was examined in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h via MTT assay. * P<0.05 vs. the non-treated group (0 μM). 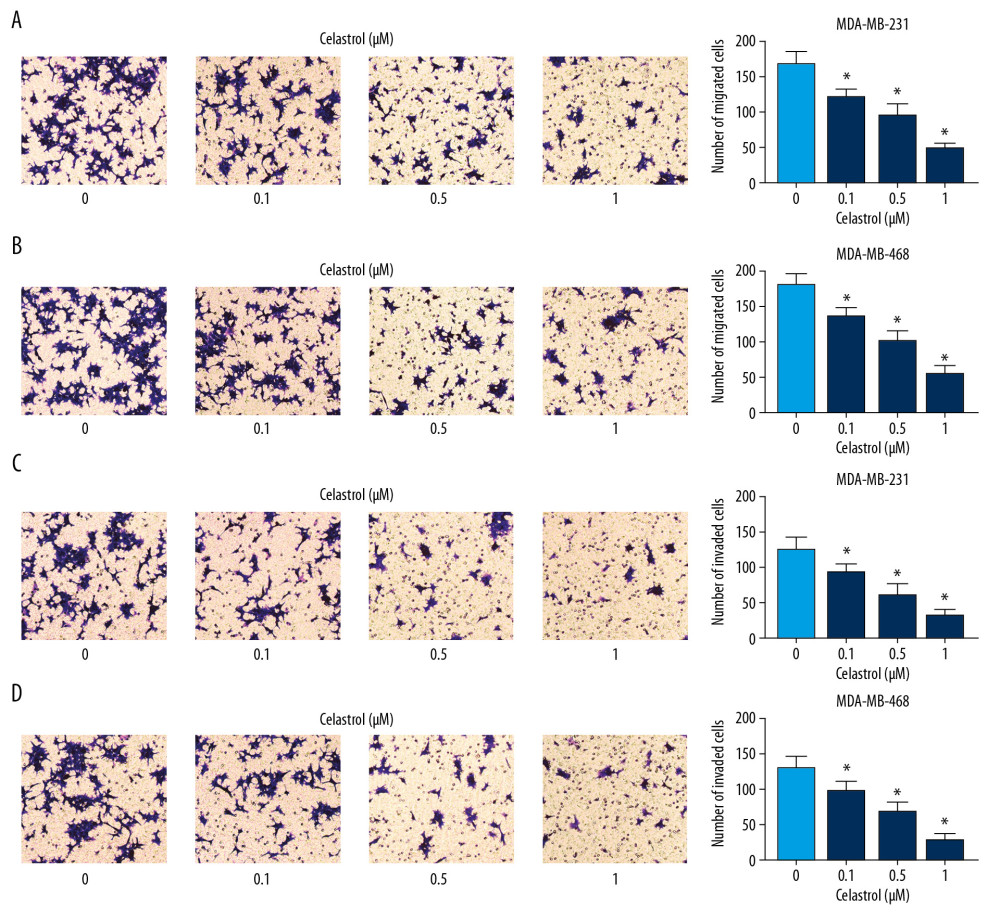 Figure 2. The influence of celastrol on migration and invasion of TNBC cells. (A, B) Cell migration was assessed in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by transwell assay. (C, D) Cell invasion was detected after treatment with various doses of celastrol for 24 h via transwell analysis. * P<0.05 vs. the non-treated group (0 μM).
Figure 2. The influence of celastrol on migration and invasion of TNBC cells. (A, B) Cell migration was assessed in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by transwell assay. (C, D) Cell invasion was detected after treatment with various doses of celastrol for 24 h via transwell analysis. * P<0.05 vs. the non-treated group (0 μM). 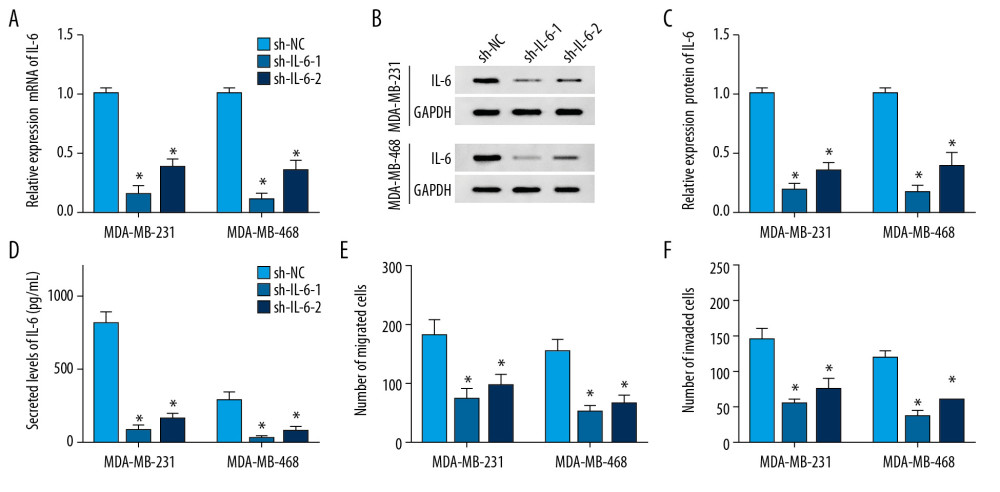 Figure 3. The influence of IL-6 inhibition on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A), protein (B) in cells and in cell medium (C) were examined in MDA-MB-231 and MDA-MB-468 cells transfected with sh-IL-6-1, sh-IL-6-2, or sh-NC by qRT-PCR, Western blot, or ELISA, respectively. (D, E) Cell migration and invasion were detected in MDA-MB-231 and MDA-MB-468 cells with transfection of sh-IL-6-1, sh-IL-6-2, or sh-NC by transwell assay. * P<0.05 vs. the negative control transfection group (sh-NC).
Figure 3. The influence of IL-6 inhibition on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A), protein (B) in cells and in cell medium (C) were examined in MDA-MB-231 and MDA-MB-468 cells transfected with sh-IL-6-1, sh-IL-6-2, or sh-NC by qRT-PCR, Western blot, or ELISA, respectively. (D, E) Cell migration and invasion were detected in MDA-MB-231 and MDA-MB-468 cells with transfection of sh-IL-6-1, sh-IL-6-2, or sh-NC by transwell assay. * P<0.05 vs. the negative control transfection group (sh-NC). 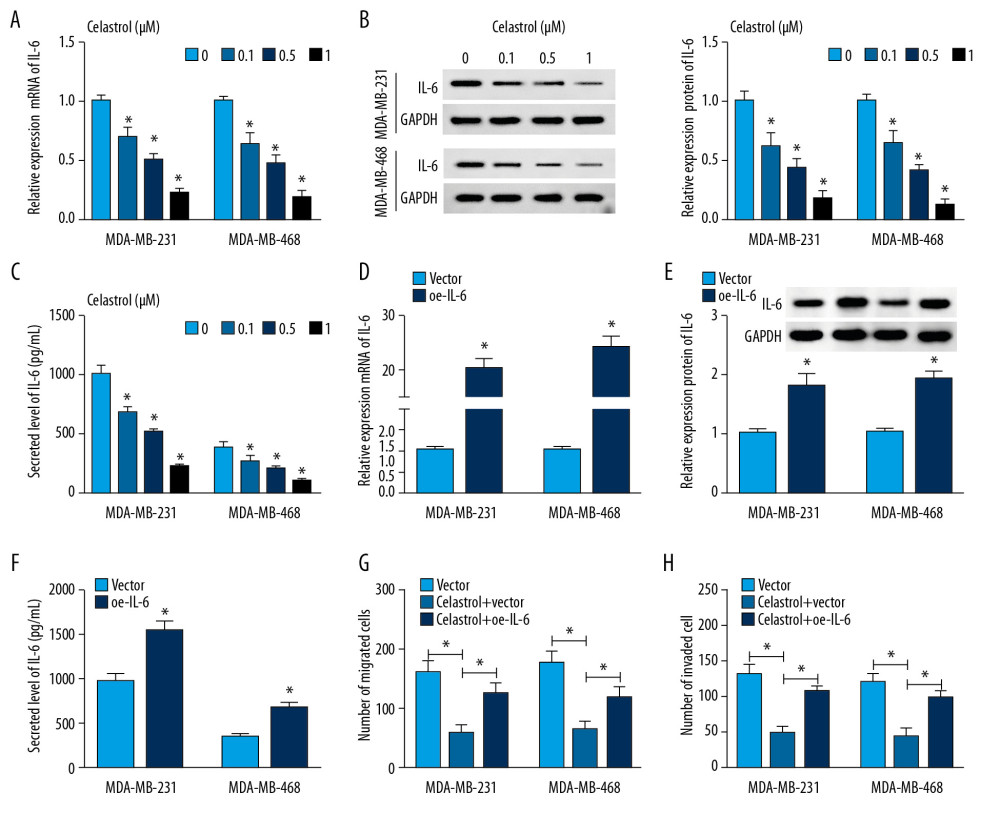 Figure 4. The effect of celastrol and IL-6 on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A) and protein (B) in cells and in cell medium (C) were measured after exposure to various doses of celastrol for 24 h by qRT-PCR, Western blot, or ELISA, respectively. (D–F) IL-6 expression was measured in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector. (G, H) Cell migration and invasion were examined in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector after treatment with celastrol. * P<0.05 vs. the corresponding control group (0 μM in A–C; vector group in D–H; celastrol+vector group in G, H).
Figure 4. The effect of celastrol and IL-6 on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A) and protein (B) in cells and in cell medium (C) were measured after exposure to various doses of celastrol for 24 h by qRT-PCR, Western blot, or ELISA, respectively. (D–F) IL-6 expression was measured in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector. (G, H) Cell migration and invasion were examined in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector after treatment with celastrol. * P<0.05 vs. the corresponding control group (0 μM in A–C; vector group in D–H; celastrol+vector group in G, H). 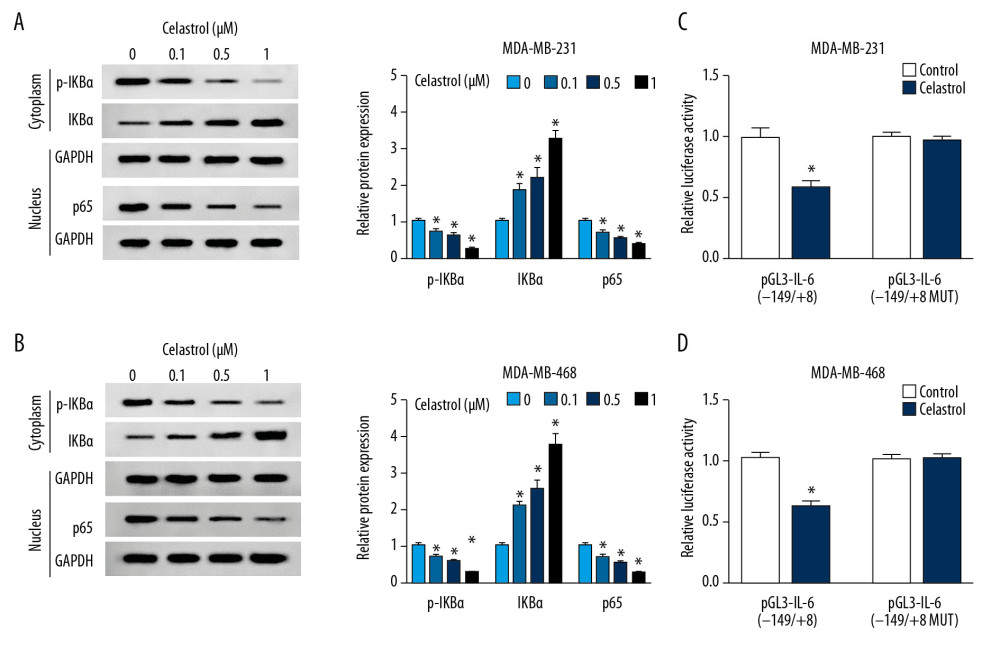 Figure 5. The effect of celastrol on NF-κB/IL-6 pathway. (A, B) The expressions of p-IKBα, IKBα, and p65 were measured in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by Western blot. (C, D) Luciferase activity of IL-6 reporter vector was analyzed in MDA-MB-231 and MDA-MB-468 cells after exposure to 1 μM celastrol for 24 h. * P<0.05 vs. the non-treated group (0 μM or control).
Figure 5. The effect of celastrol on NF-κB/IL-6 pathway. (A, B) The expressions of p-IKBα, IKBα, and p65 were measured in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by Western blot. (C, D) Luciferase activity of IL-6 reporter vector was analyzed in MDA-MB-231 and MDA-MB-468 cells after exposure to 1 μM celastrol for 24 h. * P<0.05 vs. the non-treated group (0 μM or control). References
1. Harbeck N, Gnant M, Breast cancer: Lancet, 2017; 389; 1134-50
2. Diana A, Franzese E, Centonze S, Triple-negative breast cancers: Systematic review of the literature on molecular and clinical features with a focus on treatment with innovative drugs: Curr Oncol Rep, 2018; 20; 76
3. Meirson T, Gil-Henn H, Targeting invadopodia for blocking breast cancer metastasis: Drug Resist Updat, 2018; 39; 1-17
4. Ateba SB, Mvondo MA, Ngeu ST, Natural terpenoids against female breast cancer: A 5-year recent research: Curr Med Chem, 2018; 25; 3162-213
5. Kashyap D, Sharma A, Tuli HS, Molecular targets of celastrol in cancer: Recent trends and advancements: Crit Rev Oncol Hematol, 2018; 128; 70-81
6. Li X, Zhu G, Yao X, Celastrol induces ubiquitin-dependent degradation of mTOR in breast cancer cells: Onco Targets Ther, 2018; 11; 8977-85
7. Jang SY, Jang SW, Ko J, Celastrol inhibits the growth of estrogen positive human breast cancer cells through modulation of estrogen receptor alpha: Cancer Lett, 2011; 300; 57-65
8. Kim JH, Lee JO, Lee SK, Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway: Cell Signal, 2013; 25; 805-13
9. Shrivastava S, Jeengar MK, Reddy VS, Anticancer effect of celastrol on human triple negative breast cancer: possible involvement of oxidative stress, mitochondrial dysfunction, apoptosis and PI3K/Akt pathways: Exp Mol Pathol, 2015; 98; 313-27
10. Taher MY, Davies DM, Maher J, The role of the interleukin (IL)-6/IL-6 receptor axis in cancer: Biochem Soc Trans, 2018; 46; 1449-62
11. Knupfer H, Preiss R, Significance of interleukin-6 (IL-6) in breast cancer (review): Breast Cancer Res Treat, 2007; 102; 129-35
12. Hartman ZC, Poage GM, den Hollander P, Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8: Cancer Res, 2013; 73; 3470-80
13. Okamoto M, Mizukami Y, GPER negatively regulates TNFalpha-induced IL-6 production in human breast cancer cells via NF-kappaB pathway: Endocr J, 2016; 63; 485-93
14. Liang S, Chen Z, Jiang G, Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-kappaB/IL-6 signals: Cancer Lett, 2017; 386; 12-23
15. Kim Y, Kang H, Jang SW, Celastrol inhibits breast cancer cell invasion via suppression of NF-κB-mediated matrix metalloproteinase-9 expression: Cell Physiol Biochem, 2011; 28; 175-8
16. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method: Methods, 2001; 25; 402-8
17. Tsui KH, Feng TH, Hsieh WC, Expression of interleukin-6 is downregulated by 17-(allylamino)-17-demethoxygeldanamycin in human prostatic carcinoma cells: Acta Pharmacol Sin, 2008; 29; 1334-41
18. Zhu L, Li L, Li Y, Chinese herbal medicine as an adjunctive therapy for breast cancer: A systematic review and meta-analysis: Evid Based Complement Alternat Med, 2016; 2016 9469276
19. Wang Z, Zhai Z, Du X, Celastrol inhibits migration and invasion through blocking the NF-kappaB pathway in ovarian cancer cells: Exp Ther Med, 2017; 14; 819-24
20. Wu J, Ding M, Mao N, Celastrol inhibits chondrosarcoma proliferation, migration and invasion through suppression CIP2A/c-MYC signaling pathway: J Pharmacol Sci, 2017; 134; 22-28
21. Yu X, Wang Q, Zhou X: Oncol Lett, 2016; 12; 3423-28
22. Mi C, Shi H, Ma J, Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9: Oncol Rep, 2014; 32; 2527-32
23. Li X, Wang H, Ding J, Celastrol strongly inhibits proliferation, migration and cancer stem cell properties through suppression of Pin1 in ovarian cancer cells: Eur J Pharmacol, 2019; 842; 146-56
24. Wu M, Chen W, Yu X, Celastrol aggravates LPS-induced inflammation and injuries of liver and kidney in mice: Am J Transl Res, 2018; 10; 2078-86
25. Jayatilaka H, Tyle P, Chen JJ, Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration: Nat Commun, 2017; 8; 15584
26. Fu S, Lin J, Blocking interleukin-6 and interleukin-8 signaling inhibits cell viability, colony-forming activity, and cell migration in human triple-negative breast cancer and pancreatic cancer cells: Anticancer Res, 2018; 38; 6271-79
27. Noori MS, O’Brien JD, Champa ZJ, Phenylmethimazole and a thiazole derivative of phenylmethimazole inhibit IL-6 expression by triple negative breast cancer cells: Eur J Pharmacol, 2017; 803; 130-37
28. Chiang KC, Tsui KH, Chung LC, Celastrol blocks interleukin-6 gene expression via downregulation of NF-kappaB in prostate carcinoma cells: PLoS One, 2014; 9; e93151
29. Zhang X, Zhao W, Liu X, Celastrol ameliorates inflammatory pain and modulates HMGB1/NF-κB signaling pathway in dorsal root ganglion: Neurosci Lett, 2019; 692; 83-89
30. Zhang J, Zhou K, Zhang X, Celastrol ameliorates inflammation in human retinal pigment epithelial cells by suppressing NF-κB signaling: J Ocul Pharmacol Ther, 2019; 35(2); 116-23
31. Zhao Q, Liu F, Cheng Y, Celastrol protects from cholestatic liver injury through modulation of SIRT1-FXR signaling: Mol Cell Proteomics, 2019; 18(3); 520-33
32. Wang H, Ahn KS, Alharbi SA, Celastrol alleviates gamma irradiation-induced damage by modulating diverse inflammatory mediators: Int J Mol Sci, 2020; 21(3); 1084
Figures
 Figure 1. The effect of celastrol on viability of TNBC cells. (A, B) Cell viability was examined in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h via MTT assay. * P<0.05 vs. the non-treated group (0 μM).
Figure 1. The effect of celastrol on viability of TNBC cells. (A, B) Cell viability was examined in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h via MTT assay. * P<0.05 vs. the non-treated group (0 μM). Figure 2. The influence of celastrol on migration and invasion of TNBC cells. (A, B) Cell migration was assessed in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by transwell assay. (C, D) Cell invasion was detected after treatment with various doses of celastrol for 24 h via transwell analysis. * P<0.05 vs. the non-treated group (0 μM).
Figure 2. The influence of celastrol on migration and invasion of TNBC cells. (A, B) Cell migration was assessed in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by transwell assay. (C, D) Cell invasion was detected after treatment with various doses of celastrol for 24 h via transwell analysis. * P<0.05 vs. the non-treated group (0 μM). Figure 3. The influence of IL-6 inhibition on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A), protein (B) in cells and in cell medium (C) were examined in MDA-MB-231 and MDA-MB-468 cells transfected with sh-IL-6-1, sh-IL-6-2, or sh-NC by qRT-PCR, Western blot, or ELISA, respectively. (D, E) Cell migration and invasion were detected in MDA-MB-231 and MDA-MB-468 cells with transfection of sh-IL-6-1, sh-IL-6-2, or sh-NC by transwell assay. * P<0.05 vs. the negative control transfection group (sh-NC).
Figure 3. The influence of IL-6 inhibition on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A), protein (B) in cells and in cell medium (C) were examined in MDA-MB-231 and MDA-MB-468 cells transfected with sh-IL-6-1, sh-IL-6-2, or sh-NC by qRT-PCR, Western blot, or ELISA, respectively. (D, E) Cell migration and invasion were detected in MDA-MB-231 and MDA-MB-468 cells with transfection of sh-IL-6-1, sh-IL-6-2, or sh-NC by transwell assay. * P<0.05 vs. the negative control transfection group (sh-NC). Figure 4. The effect of celastrol and IL-6 on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A) and protein (B) in cells and in cell medium (C) were measured after exposure to various doses of celastrol for 24 h by qRT-PCR, Western blot, or ELISA, respectively. (D–F) IL-6 expression was measured in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector. (G, H) Cell migration and invasion were examined in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector after treatment with celastrol. * P<0.05 vs. the corresponding control group (0 μM in A–C; vector group in D–H; celastrol+vector group in G, H).
Figure 4. The effect of celastrol and IL-6 on migration and invasion of TNBC cells. The levels of IL-6 mRNA (A) and protein (B) in cells and in cell medium (C) were measured after exposure to various doses of celastrol for 24 h by qRT-PCR, Western blot, or ELISA, respectively. (D–F) IL-6 expression was measured in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector. (G, H) Cell migration and invasion were examined in MDA-MB-231 and MDA-MB-468 cells with transfection of oe-IL-6 or vector after treatment with celastrol. * P<0.05 vs. the corresponding control group (0 μM in A–C; vector group in D–H; celastrol+vector group in G, H). Figure 5. The effect of celastrol on NF-κB/IL-6 pathway. (A, B) The expressions of p-IKBα, IKBα, and p65 were measured in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by Western blot. (C, D) Luciferase activity of IL-6 reporter vector was analyzed in MDA-MB-231 and MDA-MB-468 cells after exposure to 1 μM celastrol for 24 h. * P<0.05 vs. the non-treated group (0 μM or control).
Figure 5. The effect of celastrol on NF-κB/IL-6 pathway. (A, B) The expressions of p-IKBα, IKBα, and p65 were measured in MDA-MB-231 and MDA-MB-468 cells after exposure to various doses of celastrol for 24 h by Western blot. (C, D) Luciferase activity of IL-6 reporter vector was analyzed in MDA-MB-231 and MDA-MB-468 cells after exposure to 1 μM celastrol for 24 h. * P<0.05 vs. the non-treated group (0 μM or control). In Press
06 Mar 2024 : Clinical Research
Comparison of Outcomes between Single-Level and Double-Level Corpectomy in Thoracolumbar Reconstruction: A ...Med Sci Monit In Press; DOI: 10.12659/MSM.943797
21 Mar 2024 : Meta-Analysis
Economic Evaluation of COVID-19 Screening Tests and Surveillance Strategies in Low-Income, Middle-Income, a...Med Sci Monit In Press; DOI: 10.12659/MSM.943863
10 Apr 2024 : Clinical Research
Predicting Acute Cardiovascular Complications in COVID-19: Insights from a Specialized Cardiac Referral Dep...Med Sci Monit In Press; DOI: 10.12659/MSM.942612
06 Mar 2024 : Clinical Research
Enhanced Surgical Outcomes of Popliteal Cyst Excision: A Retrospective Study Comparing Arthroscopic Debride...Med Sci Monit In Press; DOI: 10.12659/MSM.941102
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








