28 June 2020: Articles 
Irinotecan-Associated Dysarthria in Patients with Pancreatic Cancer: A Single Site Experience
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Unexpected drug reaction, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Ali Elbeddini1ABCDEF*, Naushin Hooda2ACDF, Mohamed Gazarin3BF, Penny Webster4BE, Jackie McMillan4BDOI: 10.12659/AJCR.924058
Am J Case Rep 2020; 21:e924058
Abstract
BACKGROUND: Irinotecan, a topoisomerase I inhibitor, is a cytotoxic chemotherapeutic agent used to treat multiple malignancies, including those of colorectal, pancreatic, cervical, esophageal, gastric, and lung origin. Dysarthria, a state of difficult or unclear articulation of speech, has been reported as a rare side effect of irinotecan through multiple case reports and case series, but with limited published data aimed at understanding the underlying mechanism and effective management strategies.
CASE REPORT: We describe herein 3 cases of patients with pancreatic malignancy who experienced dysarthria while being treated with a chemotherapy regimen containing irinotecan at an ambulatory outpatient satellite chemotherapy site. All patients described received first-line FOLFIRINOX for pancreatic cancer and experienced dysarthria during their first infusion of irinotecan. In all cases, dysarthria was observed as a transient adverse drug reaction within the first 10 to 70 min of irinotecan infusion, which resolved rapidly upon pausing infusion without any long-term sequalae. All patients remained conscious and alert; physical and neurological examinations at dysarthria onset revealed no abnormalities. Some patients experienced distal extremity paresthesia, a known manifestation of oxaliplatin-induced acute neurotoxicity, and diaphoresis and nausea. Increased infusion time effectively prevented dysarthria during subsequent infusions.
CONCLUSIONS: Oncologists, pharmacists, nurses, and other care team members should be aware that irinotecan-associated dysarthria is a rare, mild, and self-limiting phenomenon to avoid inadvertently altering or withholding therapy. We suggest extending irinotecan infusion time, as opposed to dose reduction or treatment discontinuation, as a practical clinical management strategy for patients who develop recurrent dysarthria secondary to irinotecan infusion.
Keywords: Drug-Related Side Effects and Adverse Reactions, Pancreatic Neoplasms, dysarthria, irinotecan, Topoisomerase I Inhibitors
Background
Irinotecan, a semisynthetic cytotoxic alkaloid extracted from plants such as Camptotheca acuminata, is a commonly used cytotic chemotherapy agent used in the treatment of multiple malignancies, particularly of gastrointestinal origin [1–3]. Irinotecan is a prodrug that requires
Pancreatic cancer is expected to be the third leading cause of Canadian cancer deaths in 2020, surpassing breast cancer [8]. In its treatment, irinotecan can be combined with 5-fluorouracil and leucovorin alone (FOLFIRI) or in combination also with oxaliplatin (FOLFIRINOX or FOLFOXIRI) [9].
FOLFIRINOX, a combination of folinic acid (leucovorin), fluorouracil, irinotecan, and oxaliplatin, is the first-line treatment of locally advanced unresectable or metastatic pancreatic adenocarcinoma in patients who have good performance status, according to Cancer Care Ontario (CCO) guidelines [10]. Oxaliplatin 85 mg/m2 is administered IV over 2 h, followed by leucovorin 400 mg/m2 IV over 2 h [10]. Thirty minutes after starting leucovorin, irinotecan 180 mg/m2 is given IV over 90 min concurrently with the leucovorin. Intravenous fluorouracil 400 mg/m2 bolus is given after leucovorin, followed by a 46-h continuous IV infusion at a dose of 2400 mg/m2 [10]. This cycle is repeated every 2 weeks until the disease progresses or unacceptable toxicity occurs [10]. These toxicities may include early and delayed diarrhea, myelosuppression, and cholinergic syndrome, which frequently lead to dose reduction or treatment interruption [4,11,12].
Dysarthria, as defined by the American Speech-Language-Hearing Association, is a motor speech disorder resulting from impaired movement of muscles in the lips, tongue, vocal folds, or diaphragm, and is characterized by problems related to articulation, breathing, and phonation [13]. Identified through postmarketing surveillance, it is reflected in the drug monograph as an uncommon adverse drug reaction (ADR) in patients receiving irinotecan and has been reported in multiple case reports in the literature as occurring secondary to irinotecan infusion [4,14–22]. However, the precise pathogenesis of this neurotoxic effect and optimal management strategies have yet to be elucidated.
Case Reports
PATIENT (A):
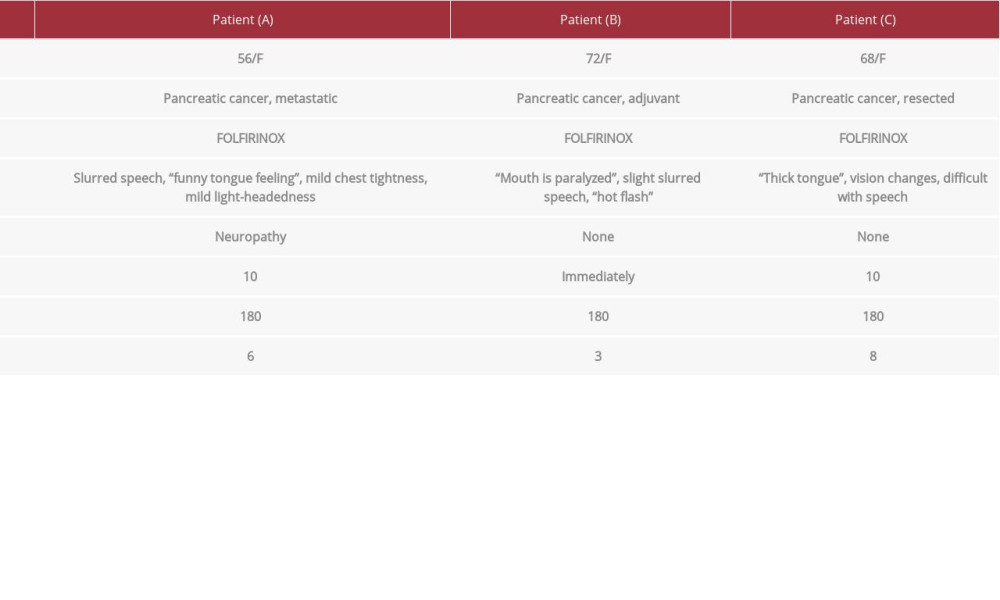
Patient A was a 56-year-old female patient with metastatic pancreatic cancer who presented to the clinic for first-line treatment with FOLFIRINOX with palliative intent. A computerized tomography (CT) scan completed before treatment initiation showed a hypodense mass of 41×26 mm in the pancreatic body with likely involvement of the celiac axis, common hepatic artery, and splenic artery. She had no other known medical conditions, no smoking history, and no allergies or previously recorded ADRs or intolerances to medications. The pre-chemotherapy biochemical and blood count (CBC) results were within normal range. The Karnofsky Performance Scale was 90 (Eastern Cooperative Oncology Group Performance Status equivalent, 0), indicating the patient was able to carry on normal activity with minor signs or symptoms of the disease.
During the first cycle of FOLFIRINOX, administered as per CCO guidelines, the patient reported slurred speech 10 min after the start of the irinotecan infusion, which the patient described as having a “funny tongue” feeling, accompanied by mild chest tightness and light-headedness. Infusion was paused until symptoms resolved 15 min later, without the need for additional hypersensitivity medications. The patient remained alert throughout the infusion. Physical and neurological examinations, including vital signs, conducted at the first onset of dysarthria revealed no additional abnormalities.
The patient was re-challenged at ¼ rate over 360 min. A trial increase in rate to ½ (over 180 min) resulted in symptom recurrence including slurred speech, chest tightness, and dizziness. The electrocardiogram did not reveal any abnormal findings. Intravenous diphenhydramine 50 mg and methylpredniso-lone 125 mg were administered, and provided symptom relief. Irinotecan was restarted at ¼ rate for 15 min, increased to ½ rate for 15 min, and then resumed to the regular rate for the remainder. Although there was no reoccurrence of dysarthria, nausea and vomiting occurred resulting in the administration of metoclopramide 10 mg IV.
During the second cycle, treatment was better tolerated with mild dysarthria during the irinotecan infusion rate of 90 min with the absence of other symptoms. Following this, the care team decided to increase the infusion time from 90 min to 180 min for the third cycle. In addition to this change, the oxaliplatin dose was reduced to 75% of the first dose due to neuropathy. The patient began exhibiting difficulty articulating words 90 min after the infusion started, although the patient described this as less prominent than the previous cycle. Upon physical examination, no swelling or shortness of breath was noted. Symptoms resolved within 30 min of going home.
During the fourth cycle, Irinotecan was administered over 180 min. The patient noted having slurred speech and nausea despite pre-treatment with dexamethasone 12 mg and diphenhydramine 50 mg IV. Irinotecan infusion was paused, and metoclopramide 10 mg IV was given as per medical directive, and symptom resolution occurred within 15 min. The patient was discharged with mild slurring, which was less pronounced than in the first treatment, and the patient stated it resolved in less than 1 h.
During the fifth cycle, some slurred speech was noticed halfway through the infusion despite receiving premedication (diphenhydramine infusion was given over 3 h). Treatment was tolerated well. The patient continued to have mildly slurred speech at the end of the infusion.
After a 4-month chemotherapy holiday, the patient resumed irinotecan infusion, which was administered over 180 min with 50 mg IV dimenhydrinate administered as a pre-medication. Mild speech changes were noted at the end of treatment, although the patient reported feeling well otherwise. Vitals showed stable conditions.
PATIENT (B):
Patient B was a 72-year-old female patient with metastatic pancreatic cancer who presented to the clinic for first-line FOLFIRINOX treatment with palliative intent. She had no history of drug allergies or ADRs. The patient’s complete biochemical and CBC results administered before the start of chemo-therapy were within normal range.
For the patient’s first cycle, irinotecan was dosed according to CCO guidelines with no prophylactic atropine due to preexisting treatment-resistant constipation and concern that atropine may worsen this. After 70 min of infusion, the patient reported that her “mouth [was] paralyzed” and that she was having a sensation like a hot flash; slightly slurred speech was noted. The irinotecan infusion was stopped until symptoms resolved 15 min later. The infusion was resumed at ½ rate (over 180 min) with no further speech changes. Vitals remained stable. The remaining treatment was tolerated well apart from some neuropathy that occurred intermittently post-treatment.
During the second cycle, irinotecan was given at a reduced rate over 225 min due to the slurred speech that occurred during the previous treatment and the patient’s anxiety about experiencing a similar sensation during this treatment. This resulted in no further incidence of slurred speech. During the third cycle, the oncologist recommended a trial of irinotecan to be administered over 180 min. No speech changes were noted.
PATIENT (C):
Patient C was a 68-year-old female diagnosed with moderately differentiated adenocarcinoma of the distal pancreas with negative margins and positive LV1/perineural invasion. A CT scan of the abdomen and pelvis revealed a 3.7×2.1 cm low-density lesion arising from the pancreatic tail along with a 24×18 cm multiloculated cyst in the mesentery with internal septations. There was no evident metastasis. She received 12 cycles of FOLFIRINOX with adjuvant intent.
While the patient was receiving the irinotecan infusion as per guidelines during her first cycle, she reported a change in vision and difficulty with speech and swallowing 20 min after infusion start, which was described by the patient as a “thick tongue” sensation. No changes were made at this time, and the infusion was continued. The patient noted this sensation lasted for 3 to 4 h after the completion of chemotherapy and had attributed it to atropine. Vital signs were stable and neurological assessments revealed no abnormalities. The care team discussed the option to hold atropine for the next treatment and only administer reactively if abdominal cramping or diarrhea was present. In addition to the dysarthria, the patient had been experiencing troublesome neuropathy in the fingers and toes. Unlike the dysarthria which resolved, the neuropathy had not improved upon the completion of chemotherapy.
For the second cycle, atropine was held. Slurred speech was noticed 30 min after the start of the irinotecan infusion, and the patient expressed difficulty in articulating words; however, this episode was less severe than during the previous treatment. No changes in vision were expressed and vitals were stable. The infusion was stopped, and symptoms resolved within 10 min. The infusion was resumed at ½ rate (over 180 min) with some return of slurred speech at the end of the infusion. The patient noted that the slurred speech was not as severe as it was during the previous treatment, and it resolved soon after the patient left the department.
For the third cycle, irinotecan was run over 180 min as ordered by the oncologist, with no incidence of speech changes. For the fourth cycle, irinotecan was trialed over 150 min as per oncologist orders, and the patient began experiencing slightly slurred speech after 10 min of irinotecan infusion. This prompted a change back to 180 min, with minimum speech changes by the end of the infusion. This rate was maintained for subsequent treatments.
Discussion
In the present study, we reported 3 cases of rare, transient dysarthria occurring in patients undergoing first-line irinotecan-containing FOLFIRINOX chemotherapy for pancreatic cancer. Table 1 provides a summary of the 3 patients with irinotecan-associated dysarthria.
Episodes of dysarthria were described by patients in many ways, including the sensation of a “thick” or “funny” tongue, as well as experiencing difficulty swallowing and enunciating words. These symptoms were objectively described by attending clinicians as slurred speech. In all cases, dysarthria was observed as a transient ADR occurring within the first 10 to 70 min of irinotecan infusion. All patients remained alert and conscious, and neurological examinations at the onset of dysarthria revealed no abnormalities, which is in line with previously reported cases [4,15–21]. A range of time-to-onset of dysarthria has been reported in the literature from occurring shortly after start of infusion to several h after infusion [15–16]. Further, other cases described in the literature ranged from 15 min to 24 h in duration [4,17,18]. Of note, there is some heterogeneity in cases described in terms of diagnoses, with most cases occurring in metastatic colorectal carcinomas, as well as in doses and duration of initial infusion, ranging from 80 to 350 mg/m2 and 30 to 120 min, respectively [4,15–21].
Identification of the causative agent of dysarthria was based on the time-to-event relationship between irinotecan infusion and dysarthria. It has been hypothesized that irinotecan-associated dysarthria can be exacerbated by concurrent administration of oxaliplatin, a platinum compound in FOLFIRINOX with known neurotoxic side effects; however, dysarthria has also occurred in patients treated with non-oxaliplatin containing regimens such as FOLFIRI, and has never been described with oxaliplatin-only regimens [19]. This suggests that irinotecan is the primary drug responsible for the dysarthria. However, as proposed by Matsuoka et al., it is plausible that FOLFIRINOX-associated dysarthria is associated with the sequence of drug administration (i.e., IV infusion of oxaliplatin, immediately followed by irinotecan), wherein the initial treatment of oxaliplatin may prime the occurrence of dysarthria [20]. This is based on the relative lack of case reports seen for FOLFOXIRI in which irinotecan is administered prior to oxaliplatin.
The frequency of dysarthria in patients undergoing FOLFIRINOX treatment has been widely reported in the literature. Gunturu et al. reported 9 cases of dysarthria occurring in a US hospital among 35 patients (25.7%) during irinotecan administration with symptoms including dysarthric speech, facial or perioral paresthesia, leg cramps, ataxia and blepharospasm. A phase II trial in Japan reported 5 cases among 36 patients (13.8%) [21,22]. Further, a hospital in Japan reported 4 cases of dysarthria among 9 patients receiving FOLFIRINOX (44.4%) [20]. These differences highlight a need to characterize risk factors for irinotecan-associated dysarthria, such as the possible role of ethnicity.
The mechanism by which central nervous system toxicity occurs after irinotecan infusion is not well understood. However, cholinergic toxicity has been proposed as a possible mechanism [4,16]. It has been proposed that irinotecan and its metabolite increase cholinergic activity by binding to the active site of the acetylcholinesterase, resulting in its functional inhibition [23]. The hypoglossal nerve, which has a role in speech function through its innervation of tongue muscles, has a higher density of cholinergic receptors compared to other brainstem nuclei, resulting in an increased intrinsic sensitivity to cholinergic stimulation [20]. Further, the density of cholinergic receptors is relatively high in the brainstem nuclei and the application of cholinergic agents results in a large increase in hypoglossal nerve activity, which innervates the tongue muscles, resulting in overstimulation manifested as dysarthria [20]. Based on this hypothesis, atropine would be a reasonable medical intervention; however, when administered at therapeutic doses, it has minimal effects on the central nervous systems [20]. Other agents such as scopolamine, with increased central nervous system activity, may have a role in managing irinotecan-induced dysarthria [18]. Further, predictive factors for the development of cholinergic syndrome, as determined by an ordered logistic regression analysis conducted by Kanbayashi et al., may shed light on predictive factors of this toxicity including female sex, and irinotecan dose ≥175 mg [24].
In further support of the cholinergic hypothesis, patients described in the present study also experienced classical cholinergic symptoms including rhinitis, acute-onset diarrhea, and abdominal pain. One of the 3 patients in this study experienced diaphoresis, described by the patient as a “hot flash.” Known symptoms of cholinergic syndrome include rhinitis, hypersalivation, miosis, lacrimation, diaphoresis, flushing, diarrhea, and abdominal cramping [18,20].
There have been a few successful management strategies, primarily based on case reports, reported in the literature. Gunturu et al. presented patient cases wherein symptoms remitted with interruption and dose reduction of the irinotecan infusion, with some recurrence upon re-challenging. With recurrence, administration of anticholinergic agents (atropine and/or diphenhydramine) resulted in complete symptomatic recovery [21]. This was similarly seen in case study reported by Lee et al. wherein dysarthria disappeared with the discontinuation of irinotecan therapy and reappeared with subsequent administration of irinotecan [25]. Matsuoka et al. presented case reports ofhaving prevented or alleviated symptoms with intramuscular atropine administration and further described a management option of utilizing atropine prophylactically as a prevention strategy, presumingly due its antagonistic action of the acetylcholine receptor [20]. However, this strategy has failed to demonstrate benefit in other studies [4,25,26].
In the present study, where 2 out of the 3 patients did not use atropine due to refusal or constipation concerns, extending the infusion time of irinotecan to at least 180 min was effective in preventing reoccurrence. This observed correlation suggests a pharmacokinetic relationship may exist, whereby irinotecan or its metabolite may accumulate more rapidly when infused over a shorter period of time, resulting in toxicity. This hypothesis has been explored through investigations of irinotecan and SN-38 accumulation in plasma levels; however, accumulation in the cerebrospinal fluid has yet to be explored [18,26]. One pharmacokinetic study conducted in a nonhuman primate model concluded that the level of irinotecan in the cerebrospinal fluid was 14% of the plasma level [27]. Further human pharmacokinetic analysis studying the peak cerebrospinal fluid concentrations of irinotecan and its metabolite, SN-38, with changes in infusion duration may evaluate this hypothesis.
Conclusions
Irinotecan-associated dysarthria, as a rare adverse effect, was reported with all 3 patients during the drug administration. Neurological manifestations included changes in speech, difficulty swallowing and enunciating words, and subjective remarks of having a “thick tongue.” The onset of dysarthria ranged from 10 to 70 min. No long-term sequelae or progression on CT scan were noted, and all cases were reversible using various management measures, including increasing infusion time to 180 min and pausing infusion for 5 to 15 min followed by a re-challenge. Limitations of our study included the small number of patients in our series and the retrospective study design. Prospective trials and pharmacokinetic studies are needed to validate the efficacy of extending infusion time to prevent irinotecan-associated dysarthria, as well as to identify probable pre-disposing factors.
References:
1.. : Drug Name: Irinotecan Monograph, 2018, British Columbia, BC Cancer Available from: http://www.bccancer.bc.ca/drug-databasesite/Drug%20Index/Irinotecan_monograph.pdf
2.. O’Reilly S, Rowinsky E, The clinical status of Irinotecan (CPT-11), a novel water-soluble camptothecin analogue: Crit Rev Oncol/Hemat, 1996; 24; 47-70
3.. : Irinotecan; 2020, Ontario, Cancer Care Ontario Available from: https://www.cancercareontario.ca/en/node/44181
4.. Dressel A, van der Mijn J, Aalders I, Irinotecan-induced dysarthria: Case Rep Oncol, 2012; 5; 47-51
5.. Klein CE, Gupta E, Reid JM, Population pharmacokinetic model for Irinotecan and two of its metabolites, SN-38 and SN-38 glucuronide: Clin Pharmacol Ther, 2002; 72; 638-47
6.. Robinson M, Osheroff N, Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II: Biochemistry, 1991; 30; 1807-13
7.. Singh RJ, Rajpoot N, Quantification of irinotecan (CPT-11) and its metabolite, SN-38, in rat plasma and bile samples: Application to pharmacokinetic studies: World J Pharml Res, 2018; 7; 1546-78
8.. Brenner D, Weir H, Demers A, Projected estimates of cancer in Canada in 2020: Can Med Assoc J, 2020; 192; 199-205
9.. Taïeb J, Lecomte T, Aparicio T, FOLFIRI.3, a new regimen combining 5-fluorouracil, folinic acid and irinotecan, for advanced pancreatic cancer: results of an Association des Gastro-Entérologues Oncologues (Gastroenterologist Oncologist Association) multicenter phase II study: Ann Oncol, 2007; 18; 498-503
10.. : FOLFIRINOX, 2020, Ontario, Cancer Care Ontario Available from: https://www.cancercareontario.ca/en/drugformulary/regimens/monograph/46436
11.. Hecht JR, Gastrointestinal toxicity of irinotecan: Oncology, 1998; 12; 73-78
12.. Adeyinka A, Kondamudi NP: Cholinergic crisis, 2020, Treasure Island (FL), StatPearls
13.. : Dysarthria, 2020, Rockville, MD, ASHA Available from: https://www.asha.org/public/speech/disorders/dysarthria/
14.. : Drug Monograph: Irinotecan, 2019, Kirland, Quebec, ASHA Available from: https://www.pfizer.ca/sites/default/files/201903/Irinotecan_PM_E_224794_06Mar2019.pdf
15.. Garcia IS, Rueda A, Alba E, Irinotecan-induced central nervous system toxicity: A case report: J Natl Cancer Inst, 1999; 91; 647
16.. Gomez JA, Sanches I, Ramirez JA, Irinotecan-induced dysarthria: An insight into its pathogenesis?: Gastrointest Cancer Res, 2008; 2(4); 209-10
17.. De Marco S, Squilloni E, Vigna L, Irinotecan chemotherapy associated with transient dysarthria and aphasia: Ann Oncol, 2004; 15; 1147-48
18.. Ramirez K, Koch M, Edenfield W, Irinotecan-induced dysarthria: A case report and review of the literature: J Oncol Pharm Pract, 2016; 23; 226-30
19.. Zhen DB, McDevitt RL, Zalupski MM, Sahai V, Irinotecan-associated dysarthria: A single institution case series with management implications in patients with gastrointestinal malignancies: J Oncol Pharm Pract, 2018; 25; 980-86
20.. Matsuoka A, Maeda O, Inada-Inque M, FOLFIRINOX-induced reversible dysarthria: A case report and review of previous cases: Oncol Lett, 2015; 10; 2662-64
21.. Gunturu KS, Yao X, Cong X, FOLFIRINOX for locally advanced and metastatic pancreatic cancer: Single institution retrospective review of efficacy and toxicity: Med Oncol, 2013; 30; 361
22.. Okusaka T, Ikeda M, Fukutomi A, Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer: Cancer Sci, 2014; 105; 1321-26
23.. Blandizzi C, De Paolis B, Colucci R, Characterization of a novel mechanism accounting for the adverse cholinergic effects of the anticancer drug irinotecan: Br J Pharmacol, 2001; 132; 73-84
24.. Kanbayashi Y, Ishikawa T, Kanazawa M, Predictive factors for the development of irinotecan-related cholinergic syndrome using ordered logistic regression analysis: Med Oncol, 2018; 35; 82
25.. Lee K, Kang HW, Ahn JH, Dysarthria induced by irinotecan in a patient with colorectal cancer: Am J Health Syst Pharm, 2013; 70; 1140-43
26.. Hamberg P, De Jong FA, Brandsma D, Irinotecan-induced central nervous system toxicity: Report on two cases and review of the literature: Acta Oncol, 2008; 47; 974-78
27.. Blaney SM, Takimoto C, Murry DJ, Plasma and cerebrospinal fluid pharmacokinetics of 9-aminocamptothecin (9-AC), irinotecan (CPT-11), and SN-38 in nonhuman primates: Cancer Chemother Pharmacol, 1998; 41; 464-68
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250