11 November 2020: Articles 
Cardiac Amyloidosis Detected on Imaging of Patients with Heart Failure
Challenging differential diagnosis, Unusual setting of medical care, Rare disease
Siniša Roginić12ABCDEF*, Ozren Vinter3ADEF, Matias Trbušić3CDEF, Martina Roginić12ABDEF, Edina Ćatić Ćuti4ADEFDOI: 10.12659/AJCR.926290
Am J Case Rep 2020; 21:e926290
Abstract
BACKGROUND: Amyloidosis is a multisystem disease caused by deposition of dysfunctional protein-amyloid-in various organs. The heart is commonly involved, especially in primary (AL) and transthyretin (ATTR) amyloidosis. Most patients present with restrictive cardiomyopathy along with other systemic features of amyloid deposition. Diagnosing amyloidosis is cumbersome and based on the patient’s clinical condition and findings from electrocardiography, routine laboratory tests, cardiac biomarkers, imaging, and biopsy. Echocardiography (echo) is a widely available diagnostic imaging method that can help raise suspicion of cardiac amyloidosis (CA) if novel parameters of systolic dysfunction are used, which are based on strain measurement complemented with traditional morphologic and hemodynamic traits. A definitive diagnosis of amyloidosis requires biopsy. It is important to differentiate between AL and ATTR amyloidosis because the treatment approaches for them differ. The prognosis for CA is still dismal but can be improved with early diagnosis and institution of treatment.
CASE REPORT: Our patients presented with advanced heart failure and subtle clinical signs of amyloidosis. AL amyloidosis was diagnosed based on echo findings and confirmed with bone marrow biopsy. In this report, we describe classic nonspecific echo signs followed by novel parameters of systolic dysfunction.
CONCLUSIONS: Because the symptoms and signs of amyloidosis are nonspecific, the diagnosis requires a high level of clinical suspicion. Severe diastolic heart failure should prompt a further search for subtle signs on echo that indicate possible amyloid deposition disease. Use of systolic strain analysis increases the specificity of echo for diagnosis of amyloidosis. Echo results combined with specific clinical symptoms and results of a hematology workup can be used to diagnose CA when other, less common tests are not available or invasive testing is not desirable.
Keywords: Amyloidosis, Cardiomyopathy, Restrictive, Echocardiography, Cardiomyopathies, Diagnostic Imaging, Prealbumin
Background
Amyloidosis is the general term for a clinical condition caused by accumulation of different precursor proteins with unstable tertiary structure clumping into insoluble amyloid fibrils, which leads to dysfunction in various organs [1]. Cardiac involvement is crucial, because when present, it is a marker of disease severity and indicator of prognosis. The 2 most common causes of clinically relevant cardiac amyloidosis (CA) are light chain amyloidosis (AL), also called primary amyloidosis, and transthyretin amyloidosis (ATTR) [2,3]. AL amyloidosis is a primary plasma cell disorder that occurs on its own or in association with multiple myeloma [4]. Immunoglobulin light chains have an additional direct toxic effect on myocytes; therefore, the clinical presentation is more severe than in ATTR amyloidosis [5,6].
The median age at diagnosis of amyloidosis is 64 years, and two-thirds of cases are in men [7]. ATTR amyloidosis results from deposition of the abnormal transport protein transthyretin, mainly in the heart and peripheral nerves. The 2 subtypes are wild-type ATTR and mutant ATTR. Wild-type ATTR CA has been increasingly recognized as a cause of heart failure with preserved ejection fraction in elderly individuals and will probably become the most common form of CA in that population.
Differentiating between AL and ATTR CA is crucial because the prognosis and therapy for them are completely different [8]. Diagnosis is based on identifying clinical signs of cardiac and extracardiac involvement and on results of laboratory tests, electrocardiography (ECG), and echocardiography (echo) in all patients with clinical suspicion of CA. A definitive diagnosis of amyloidosis (especially the AL subtype) requires histological confirmation and typing of the amyloid precursor on tissue biopsy. A biopsy of the clinically involved organ is not always necessay because most patients can be diagnosed based on analysis of an abdominal fat pad aspirate or biopsy of a minor labial salivary gland. Cardiac biomarkers (N-terminal pro b-type natriuretic peptide [NT-proBNP] and troponin) are crucial for diagnosing cardiac involvement. They can be positive even before symptoms occur, have prognostic significance, and increasingly are being used to monitor disease progression and response to treatment. Echo is the mainstay of diagnostic imaging because of its wide availability and reproducibility [2]. Numerous classic and novel echo signs are relatively specific for CA and the testing also offers prognostic information [2,9]. Additional tests, such as magnetic resonance imaging (MRI), scintigraphy with bone tracers, and genetic analysis, can be performed on an individual basis and depending on their availability. Early diagnosis is the determinant of prognosis, especially in AL amyloidosis, for which antineoplastic therapy should be administered as soon as possible [4]. The amyloidosis subtype and disease stage determine the strategy for treatment, which can include chemotherapy, stem cell transplantation (SCT), and liver transplantation [4]. We present the cases of 3 patients with advanced heart failure who were diagnosed with AL amyloidosis based on their clinical presentation and results of echo, laboratory testing, and bone marrow biopsy. We also discuss the echo parameters for diagnosis of the disease and assessment of prognosis.
Case Report
Patient 1 was admitted for newly diagnosed heart failure with high levels of natriuretic peptides and chronically elevated troponin values (Table 1). During standard treatment, he had experienced transitory aphasia without structural cerebral damage and recovered completely. ECG showed low voltage in the limb leads with a pseudoinfarction pattern in the precordial leads (Figure 1). That finding along with the patient’s clinical presentation (periorbital hematoma, thickened tongue, proteinuria below the nephrotic range, and a decreased glomerular filtration rate) and echo findings (Figure 2) were highly suspicious for amyloidosis.
Spontaneous echo contrast was detected in the man’s heart cavities, possibly explaining his recent cardioembolic cerebral event despite sinus rhythm. Monoclonal protein–immunoglobulin G (IgG) and lambda light chains were detected on further hematological workup (serum and urine protein immunofixation, serum kappa/lambda free light chain [FLC] ratio). Bone marrow biopsy confirmed the diagnosis of multiple myeloma: 20% CD138+ atypical plasma cells with Congo red-positive amyloid infiltrates. Chemotherapy was started, but unfortunately, the patient died 2 months later from refractory heart failure.
Patient 2 also presented with a first episode of mainly right-sided heart failure (NT-proBNP 10940 ng/L). She was a septuagenarian and already cachectic upon admission, with generalized edema. Echo revealed advanced-stage restrictive cardiomyopathy with significant reduction in left ventricular systolic function (LVSF) and telltale signs of potential amyloidosis (Figures 3, 4). Upon cardiac recompensation, the patient was transferred to the Hematology Department and diagnosed with multiple myeloma (IgG, lambda light chains) with amyloidosis based on bone marrow biopsy. Clinical and radiologic staging showed additional involvement of the tongue, kidney (proteinuria), and liver (enlargement and infiltration on computed tomography [CT] scan). After 3 cycles of chemotherapy, the patient died from an ischemic stroke.
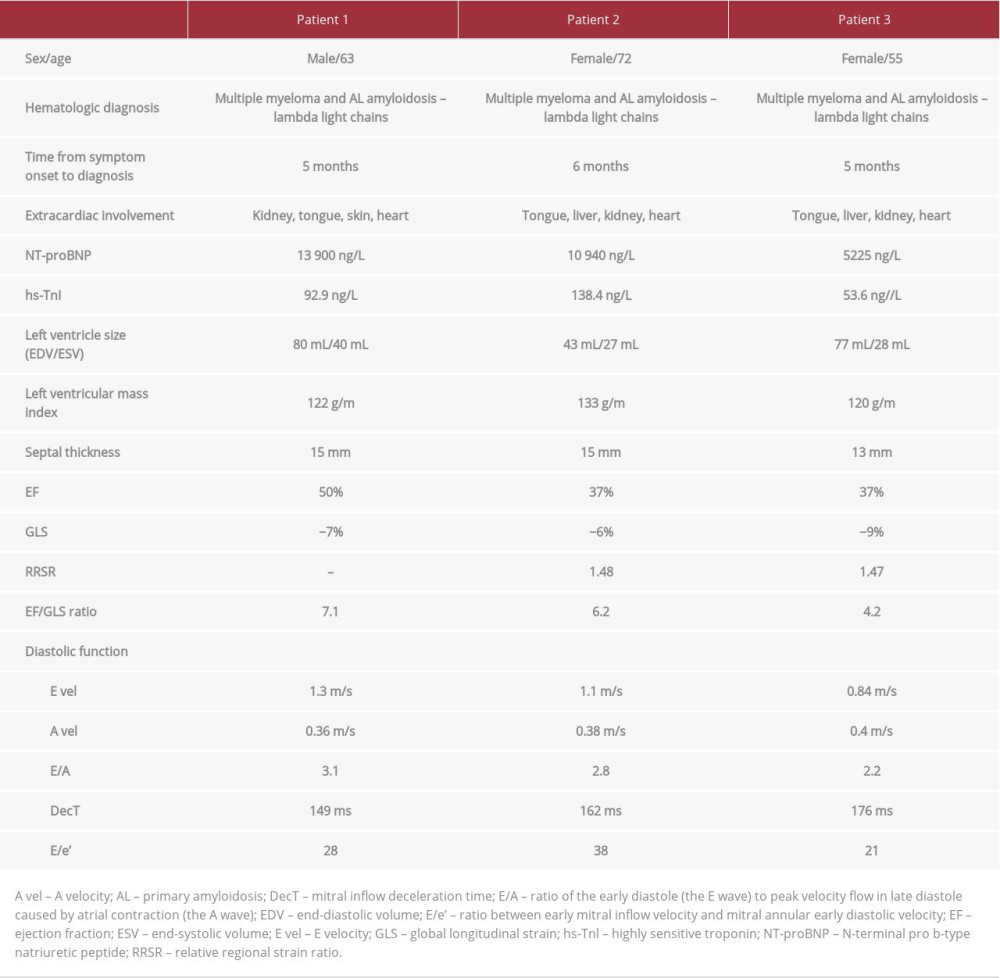
Patient 3 had a relatively short history of chronic heart failure with a mildly reduced LV ejection fraction. She deteriorated progressively despite evidence-based therapy. During her last hospitalization for worsening heart failure, a repeat echo raised suspicion for amyloidosis based on morphologic traits, the restrictive left ventricle filling pattern, and a characteristic regional reduction in longitudinal strain (Figures 5–8). One week later, the patient had a thrombosis of the inferior vena cava with a kidney infarction. Further workup fulfilled the criteria for diagnosis of multiple myeloma with amyloidosis: monoclonal lambda light chains in serum and urine electrophoresis, bone marrow infiltration with atypical plasma cells, Congo red-positive amyloid fibers, and involvement of the heart, liver, kidney, and tongue. A cardiac biopsy also was performed but the result was nondiagnostic. The patient currently is receiving chemotherapy in preparation for autologous SCT. The clinical and echo parameters for all 3 patients are summarized in Table 1.
Discussion
AL amyloidosis is fairly rare, with an incidence of 8.9 per 100 0000 and 60% of patients have cardiac involvement [7]. Our patients were initially diagnosed in a general hospital that covers a population of 120 000, so the 3 cases are a surprisingly high incidence in a short time span of 10 months. Median time to diagnosis was 5 months, while other authors have reported a median of 10 to 22 months [10,11]. The small number of patients in our report does not for allow direct comparisons; nevertheless, they were all diagnosed fairly swiftly. The median age of patients with AL amyloidosis is 60, most of whom are men [7]. The diagnosis is suspected when the clinical presentation includes classic findings, such as microvoltage and a pseudoinfarction pattern on ECG (present in all 3 of our patients) and hyperechogenic speckled myocardium on echo [12]. All of our patients had chronically mildly elevated cardiac troponin values, reflecting continuous necrosis of myocytes and significantly elevated NT-proBNP values, which correlated with the clinical picture of advanced heart failure. Cardiac biomarkers are excellent parameters for monitoring disease progression, staging, and determining prognosis. A widely used staging system from 2004 relied solely on 2 cardiac biomarkers: troponin (T or I) and NT-proBNP [13]. It was revised in 2012, adding the absolute difference in FLCs as a third variable [14].
Echo done based on a high level of clinical suspicion for the pathology was the key to further workup and achievement of definitive diagnosis in our patients. Biopsy of clinically affected organs or less invasive and, therefore, more widely used abdominal fat pad aspirate and biopsy of a salivary gland is mandatory for the diagnosis of AL amyloidosis. All of our patients had bone marrow biopsies, which showed clonal plasma cells consistent with multiple myeloma and amyloid infiltration, detected by Congo red staining. One should always keep in mind the differential diagnosis between AL and ATTR amyloidosis. For instance, ATTR amyloidosis can occur with unrelated monoclonal gammopathy, such as monoclonal gammopathy of undetermined significance, which requires follow up and no chemotherapy.
In all of our patients, the diagnosis of multiple myeloma with amyloidosis was clear and performing additional routine cardiac biopsy would not have affected treatment. Scintigraphy with technetium pyrophosphate (99mTc-PYP), which traditionally has been used as a bone tracer, has 100% specificity to differentiate between AL and ATTR CA, so it could be done to definitively exclude coexisting ATTR (which is highly unlikely) [8]. Cardiovascular MRI (cMRI) shows a typical pattern of late gadolinium ehnancement in CA, and it is suggested after echo in a CA diagnosis algorithm [1]. ATTR CA amyloidosis can even be diagnosed without biopsy. Positive scintigraphy with bone-avid tracers (Grade 2 or 3 tracer uptake) in the absence of detectable monoclonal protein in serum or urine provides a reliable nonhistological diagnosis [15]. Unfortunately, cMRI is still not widely available for routine use.
Our cases demonstrate that echo followed by a hematology workup (monoclonal protein detection and bone marrow biopsy) is a possible pathway to diagnosis of AL amyloidosis in a large number of patients, obviating the need for routine cardiac biopsy. Most patients with CA have relatively preserved LVSF on presentation. That makes diagnosis of CA even harder because most clinicians would expect isolated advanced diastolic dysfunction [16]. Unfortunately, all of our patients had advanced disease with high amyloid burden and concomitant systolic dysfunction. When myocardial amyloid burden reaches a certain threshold, cardiac damage is known to become irreversible and chemotherapy does not appear to improve outcomes [17]. Two of our 3 patients had cerebral ischemic events despite sinus rhythm. Risk for thrombosis is greatly increased with ventricular diastolic dysfunction and atrial mechanical dysfunction; therefore, anticoagulation should be considered as an option in all patients with CA [4,18].
Multiple studies have shown that strain abnormalities can be an early marker of CA, often before symptoms of congestive heart failure [4]. Most of the classic echo parameters (Figures 2, 3, 5–7, 9) are relatively nonspecific for CA [2,19]. An apical sparing pattern of longitudinal strain impairment has high sensitivity (93%) and specificity (82%) for CA [4,20]. Relative apical sparing also can be seen on 2-dimensional speckle-tracking echo in patients with more common causes of myocardial thickening, such as hypertension and aortic stenosis. Additional information can be gained by analyzing dissociation between relatively preserved left ventricular ejection fraction (LVEF) and profoundly reduced global longitudinal strain (GLS), expressed as LVEF/GLS ratio. This parameter can also be used to differentiate CA from other causes of LV hypertrophy. An EF/GLS ratio higher than 4.1 has 89.7% sensitivity and 91.7% specificity for a CA diagnosis [20,21]. Our patients met these criteria. Various prognostic factors can be assessed with echo. Increased septal thickness, E/e’ ratio (ratio between early mitral inflow velocity and mitral annular early diastolic velocity), and reduced LVEF all indicate worse prognosis regardless of the type of amyloidosis [2]. In patients with AL amyloidosis and preserved LVEF, a GLS value less than −14.81 has been shown to be a predictor of all-cause mortality [2,23]. Basal-apex gradient of strain can be expressed as relative regional strain ratio (RRSR). An RRSR higher than 1.19 also predicts higher mortality or progression to heart transplantation [2,24,25].
Conclusions
CA is the most common cause of restrictive cardiomyopathy and should be considered in all patietns with heart failure who present with preserved LVEF. The prognosis is determined by the extent of myocardial damage and total amyloid burden. Overall, it has improved consistently because of advances in diagnosis and treatment. Now even patients with stage 4 AL CA have a median survival of 5.8 months [14]. Shortening the time from symptom onset to diagnosis is the key to improving outcomes. Our cases show how full use of echo in conjunction with clinical presentation, ECG, cardiac biomarkers, M protein detection, and bone marrow biopsy can sometimes provide a definitive diagnosis of CA in patients with AL amyloidosis without the need for an invasive organ biopsy. Unfortunately, technical limitations at our center did not permit us to perform futher amyloid typing. Novel, relatively specific parameters such as apical sparing or EF/GLS ratio also should be used. Chemotherapy and autologous SCT is the treatment regimen of choice for AL amyloidosis, particularly when a patient also has heart involvement. Unfortunately, the prognostic parameters proved reliable in our patients, and only 1 was alive 6 months after diagnosis, despite treatment.
Figures
References:
1.. Fontana M, Corovic A, Scully P, Moon JC, Myocardial amyloidosis: The exemplar interstitial disease: JACC Cardiovasc Imaging, 2019; 12(11 Pt 2); 2345
2.. Agha AM, Parwani P, Guha A, Role of cardiovascular imaging for the diagnosis and prognosis of cardiac amyloidosis: Open Heart, 2018; 5(2); e000881
3.. Guan J, Mishra S, Falk RH, Current perspectives on cardiac amyloidosis: Am J Physiol Heart Circ Physiol, 2012; 302; H544-52
4.. Tuzovic M, Yang EH, Baas AS, Cardiac amyloidosis: Diagnosis and treatment strategies: Curr Oncol Rep, 2017; 19(7); 46
5.. Brenner DA, Jain M, Pimentel DR, Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in the cellular oxidant stress: Circulation, 2004; 94(8); 1008-10
6.. Guan J, Mishra S, Shi J, Stanniocalcin1 is a key mediator of amyloidogenic light chain induced cardiotoxicity: Basic Res Cardiol, 2013; 108(5); 378
7.. Shah KB, Inoue Y, Mehra MR, Amyloidosis and the heart: A comprehensive review: Arch Intern Med, 2006; 166(17); 1805-13
8.. Yamamoto H, Yokochi T, Transthyretin cardiac amyloidosis: Update on diagnosis and treatment: ESC Heart Fail, 2019; 6(6); 1128-39
9.. Falk RH, Quarta CC, Echocardiography in cardiac amyloidosis: Heart Fail Rev, 2015; 20(2); 125-31
10.. Bishop E, Brown EE, Fajardo J, Seven factors predict a delayed diagnosis of cardiac amyloidosis: Amyloid, 2018; 25(3); 174-79
11.. McCausland KL, White MK, Guthrie SD, Light chain (AL) amyloidosis: The journey to diagnosis: Patient, 2018; 11(2); 207-16
12.. Cheng Z, Zhu K, Tian Z, The findings of electrocardiography in patients with cardiac amyloidosis: Ann Noninvasive Electrocardiol, 2013; 18(2); 157-62
13.. Dispenzieri A, Gertz MA, Kyle RA, Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: A staging system for primary systemic amyloidosis: J Clin Oncol, 2004; 22; 3751-17
14.. Kumar S, Dispenzieri A, Lacy MQ, Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements: J Clin Oncol, 2012; 30; 989-95
15.. Gillmore JD, Maurer MS, Falk RH, Nonbiopsy diagnosis of cardiac transthyretin amyloidosis: Circulation, 2016; 133(24); 2404-12
16.. Cueto-Garcia L, Reeder GS, Kyle RA, Echocardiographic findings in systemic amyloidosis: Spectrum of cardiac involvement and relation to survival: J Am Coll Cardiol, 1985; 6(4); 6737-43
17.. Kristen A, Brokbals E, Aus dem Siepen F, Cardiac amyloid load: A prognostic and predictive biomarker in patients with light-chain amyloidosis: J Am Coll Cardiol, 2016; 68(1); 13-24
18.. Feng D, Edwards WD, Oh JK, Intracardiac thrombosis and embolism in patients with cardiac amyloidosis: Circulation, 2007; 116; 2420-26
19.. Mohty D, Damy T, Cosnay P, Cardiac amyloidosis: Updates in diagnosis and management: Arch Cardiovasc Dis, 2013; 106; 528-40
20.. Phelan D, Collier P, Thavendiranathan P, Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis: Heart, 2012; 98(19); 1442-48
21.. Pagourelias ED, Duchenne J, Mirea O, The relation of ejection fraction and global longitudinal strain in amyloidosis: Implications for differential diagnosis: JACC Cardiovasc Imaging, 2016; 9; 1358-59
22.. Pagourelias ED, Mirea O, Duchenne J, Echo parameters for differential diagnosis in cardiac amyloidosis: A head-to-head comparison of deformation and nondeformation parameters: Circ Cardiovasc Imaging, 2017; 10; e005588
23.. Barros-Gomes S, Williams B, Nhola LF, Prognosis of light chain amyloidosis with preserved LVEF: Added value of 2D speckletracking echocardiography to the current prognostic staging system: JACC Cardiovasc Imaging, 2017; 10; 398-407
24.. Senapati A, Sperry BW, Grodin JL, Prognostic implication of relative regional strain ratio in cardiac amyloidosis: Heart, 2016; 102; 748-54
25.. Ternacle J, Bodez D, Guellich A, Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis: JACC Cardiovasc Imaging, 2016; 9(2); 126-38
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250