17 March 2021: Articles 
Chronic Headache and Cerebral Venous Sinus Thrombosis Due to Varicella Zoster Virus Infection: A Case Report and Review of the Literature
Unusual clinical course
Laith Alamlih1ABCDEF*, Mohammad Elmourtada AbdulgayoomDOI: 10.12659/AJCR.927699
Am J Case Rep 2021; 22:e927699
Abstract
BACKGROUND: Varicella zoster virus (VZV) infection causes 2 clinically distinct forms of the disease: varicella (chickenpox) and herpes zoster (shingles). Primary VZV infection results in the diffuse vesicular rash of varicella, or chickenpox. Endogenous reactivation of latent VZV typically results in a localized skin infection known as herpes zoster, or shingles. The infection usually manifests as a self-limited disease. However, it can be associated with various neurological complications such as encephalitis, meningitis, ventriculitis, cerebellar ataxia, ischemic or hemorrhagic, and, rarely, cerebral venous sinus thrombosis (CVST). This report presents a case of cerebral venous sinus thrombosis due to varicella zoster virus infection in a 20-year-old Nepalese man who presented to the Emergency Department with headache.
CASE REPORT: A 20-year-old Nepalese male patient presented to the Emergency Department with headache of 10 day’s duration. Five days prior to that, he had a diffuse pruritic skin rash. Examination as well as serology confirmed the presence of primary varicella infection. Computed tomography (CT) and magnetic resonance venography (MRV) demonstrated CVST. Thrombophilia workup revealed a transient elevation of antiphospholipid serology. Shortly after admission, the patient had a transient seizure. He was treated with acyclovir, levetiracetam, and anticoagulation. A comprehensive literature review of similar cases was performed to establish a link between thrombotic complications and primary VZV infection and to formulate possible mechanistic pathways.
CONCLUSIONS: This report shows that primary VSV infection can be associated with vasculopathy and CVST. Physicians should recognize this serious complication, which should be diagnosed and treated without delay.
Keywords: Anticoagulants, Antiphospholipid Syndrome, Herpesvirus 3, Human, Sinus Thrombosis, Intracranial, Chickenpox, Headache Disorders, Herpes Zoster, Varicella Zoster Virus Infection, young adult
Background
Varicella zoster virus (VZV) is an Alpha-herpesvirus, which is one of the Herpesviridae subfamilies. Infection usually manifests as varicella (chickenpox) or herpes zoster, particularly in older adults [1]. Chickenpox is much more common in children and tends to have a self-limiting course. However, in adults, the disease has more complications and can even lead to death [2,3]. VZV infection is linked to various neurological complications such as encephalitis, meningitis, ventriculitis, cerebellar ataxia, ischemic or hemorrhagic stroke due to arterial vasculopathy, and, rarely, cerebral venous sinus thrombosis (CVST) [4].
CVST is an uncommon causes of stroke, and it usually affects young individuals [5]. In general, the condition is rare and has an incidence of 0.32–1.57 per 100 000 person-years, with a female-to-male ratio of 3: 1 [6,7]. There are various risk factors involved in the development of CVST, including congenital thrombophilia, use of oral contraceptives, autoimmune diseases such as Behçet’s disease, and antiphospholipid syndrome, malignancy, and infections [8 9]. Although infectious causes only account for a minority of cases in developed countries, they are of high importance as a cause of CVST in developing countries [10,11]. Infection-induced CVST attributes to 8.1–35.6% of all CVST [9,12]. Varicella infection can cause various thrombotic manifestations; however, the infection is rarely associated with CVST. This report presents a case of cerebral venous sinus thrombosis due to varicella zoster virus infection in a 20-year-old Nepalese man who presented to the Emergency Department with headache. We also provide a detailed literature review of varicella-related CVST (VrCVST).
Case Report
A 20-year-old Nepalese man presented to the Emergency Department with a headache of 10 day’s duration. The headache came in severe attacks and was dull aching in nature, mainly over the frontal area. It had no specific aggravating or relieving factors. The headache was associated with dizziness, nausea, and occasional vomiting. Five days prior to these symptoms, he developed a skin rash and a subjective fever. The patient described the rash as pruritic blister-like small lesions that started over the face and later diffused to involve the trunk and limbs. The fever was more prominent at night and lasted for 3 days. The patient denied any recent travel. However, he stated that he had contact with 3 other roommates who developed a similar skin rash, but they did not seek medical advice. He thought that his roommates had not sought medical attention. Furthermore, there was no history of visual disturbances, photophobia, weakness, seizure, or abnormal behavior. The review of systems was otherwise unremarkable. There was no past medical or surgical history of note. He was not taking any medications regularly and had no prior use of medications. The patient stated that he received childhood vaccines in Nepal, but he is not aware of the details and had no record of them. He had no family history of venous thromboembolism (VTE) or premature cardiovascular events.
On examination, his Glasgow Coma Score was 15/15, blood pressure was 140/90 mmHg, body temperature was 37°C, pulse was 80 beats per minute; respiratory rate was 18 breaths per minute, and SpO2 was 97%. There were scattered small macules, papules, and vesicles over his face and body. The lesions were not localized to a specific dermatome and were more diffused and bilateral. Most of the lesions were healed, with occasional crusted lesions distributed particularly over his trunk (Figure 1). The patient had mild neck stiffness and normal power and reflexes. Results of a sensory exam were normal. He had negative Babinski sign. Cardiac, respiratory, and abdominal exams were unremarkable.
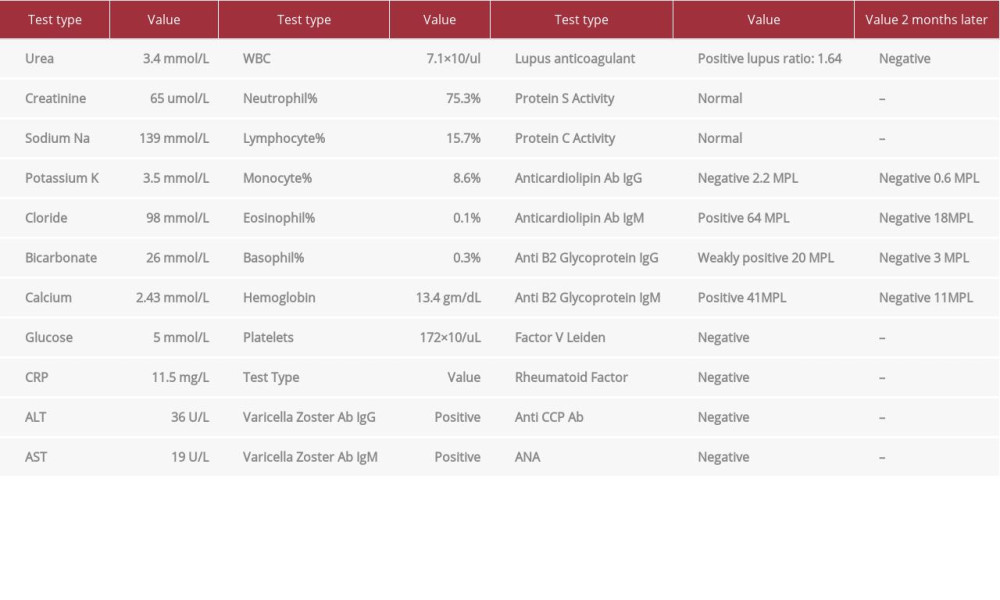
His blood investigations (Table 1) revealed a normal complete blood count, renal function, and liver function tests. However, the laboratory test results were positive for varicella zoster IgG and IgM antibodies. HIV (Human Immunodeficiency Virus) screening yielded negative results. We did not assess D-dimer or perform serology for other Herpesviridae or other viral infections. A plain computed tomography (CT) scan of the head was suggestive of CVST, so it was followed with a CT venogram (CTV), which confirmed the presence of extensive CVST involving the cerebral venous sinuses, cortical veins, and proximal left internal jugular vein (Figure 2). Magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) confirmed the finding of CVST. The images also showed a small lacunar infarction and right frontal parenchymal edema, microhemorrhages, and venous cortical-juxta cortical ischemic changes (Figure 3). However, there was no evidence of meningeal enhancement on imaging. Hypercoagulability and autoimmune workup were negative for factor V Leiden, rheumatoid factor, anti-nuclear antibodies, and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Protein C, protein S, and homocysteine levels were normal. However, the antiphospholipid syndrome (APS) workup showed a positive lupus anticoagulant, positive IgM anti-cardiolipin antibodies, positive IgM anti-b2 glycoprotein antibodies, and weakly positive IgG anti-b2 glycoprotein.
Shortly after admission, he was noticed to have attacks of brief lapse of consciousness with staring and irregular movement of his left limb, indicative of focal seizures, which raised the suspicion of the presence of meningoencephalitis. A lumbar puncture was performed successfully after multiple attempts due to inappropriate body habitus, revealing an opening pressure of 28 cmH2O, and on visual inspection, the sample was blood-tinged. Cerebrospinal fluid (CSF) analysis was done and the results showed a WBCs of 6/uL (corrected WBC for RBCs was at 4/uL) [normal range 0–5/uL], RBCs of 967/uL [normal range 0–2/uL], CSF glucose 2.91 mmol/L [normal range 2.2–3.9 mmol/L], and CSF protein 0.42 gm/L [normal range 0.15–0.45 gm/L]. The CSF was negative for gram stain, culture, acid-fast bacilli (AFB) smear, tuberculosis polymerase chain reaction (PCR), tuberculosis culture, fungal culture, cryptococcal antigen, and cytology. Due to the difficult lumbar puncture, an insufficient amount of sample was obtained to send for all the investigations. A diagnosis of primary varicella infection was made based on typical clinical manifestations, which were supported by the presence of IgM VZV antibodies. Thus, we did not need to perform an IgG antibody avidity test to differentiate acute from past infection.
The patient was stared on intravenous (IV) acyclovir 600 mg Q6 hourly, enoxaparin 80 mg subcutaneous daily (bridging), and warfarin 5 mg daily for the CVST (with an INR target of 2–3) and levetiracetam 500mg twice daily for the focal seizure. At 8 week’s follow-up, the patient was doing well and completely asymptomatic. He had stopped the warfarin after about 1 month, stating that he was feeling well and did not have reason to continue. He was counseled on the importance of taking warfarin and the possible complication of non-concordance to it. Two months later, repeated laboratory tests were unremarkable, including for the APS workup, which was negative for lupus anticoagulant as well as for APS antibodies (Table 1).
Discussion
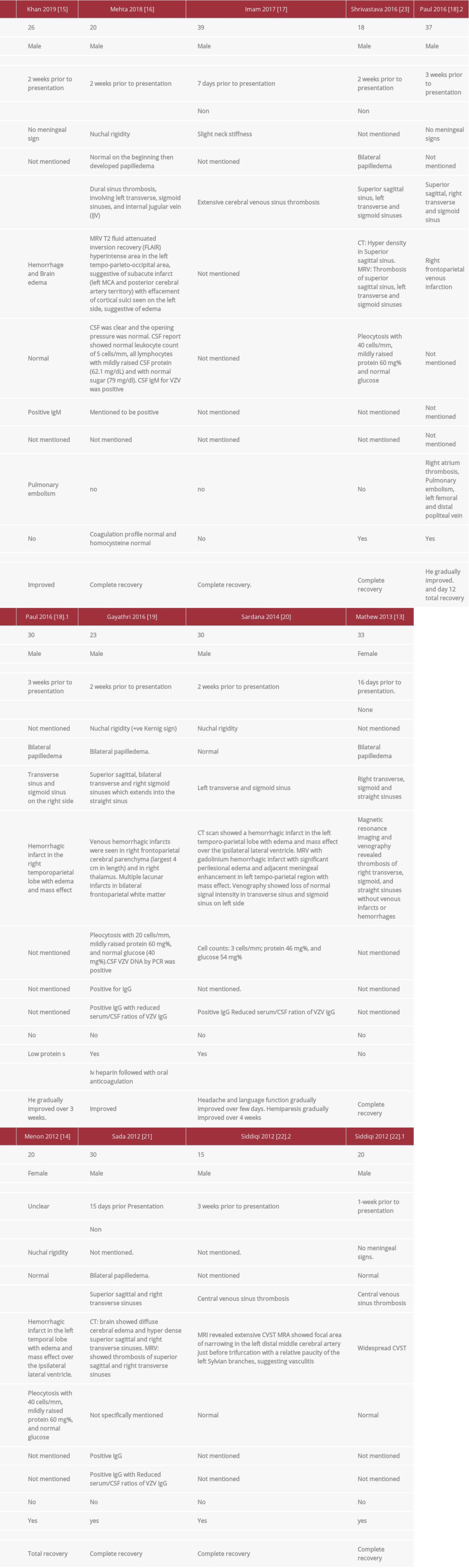
This case demonstrates that varicella infection can cause thrombosis by production of antiphospholipid antibodies. The diagnosis of primary varicella infection was made clinically and was confirmed by serology. The CVST was chronologically correlated with varicella and confirmed with MRV. The patient was found to have transient APS biomarkers; otherwise, all other common congenital and acquired causes of thrombophilia were ruled out. We performed a comprehensive literature review searching for CVST cases related to primary varicella infection. We searched 3 databases – PubMed, Embase (OVID: 1980 to current), and Scopus – to 23 June 2020. A combination of keywords limited to title/abstract field and subject terms were used in the base search. No language or date restrictions were used in any of the searches. We included all case reports of primary varicella infection-related CVST that were similar to our case. We found 11 articles describing 13 patients with VrCVST (Table 2) [13–23]. The condition was found to primarily affect males, contrary to other causes of CVST, which primarily affect females [7]. All patients were young; the age range was 15–39 years, with a mean of 25.8 years and a median of 26 years. This is consistent with the predominance of CVST in the young population. However, patients with VrCVST are younger than CVST patients [9].
Our patient developed manifestations suggestive of CVST (thunderclap headache), about 5 days after the appearance of the skin rash and fever. The CVST was diagnosed 2 weeks after he started to have symptoms. This is consistent with the reported cases, which developed CVST 2–3 weeks after the onset of VZV disease, except for 2 cases in which CVST was diagnosed after 1 week from the presentation of initial symptoms [17,22]. The headache in our patient was thunder-clap in nature. This is a severe type of headache that comes in sudden attacks, reaching a peak within 1 min from the onset. This type of headache can be seen in various diseases particularly in subarachnoid hemorrhage. Although thunder-clap headache is linked to the CVST, it was found that only a minority of CVST patients present with it. Most of the VrCVST cases developed acute headaches which is consistent with the presentation of CVST in general [24].
Our patient’s lapse of consciousness and thumb movements are suggestive of focal seizure. In our literature search, 4 out of 13 patients developed seizures [15,18,22,23]. This is compatible with CVST due to other causes, in which 30–40% of patients develop acute symptomatic seizures which occur within 2 weeks of the diagnosis [25].
The CSF analysis of our patient showed an increase in RBC counts secondary to a traumatic lumbar puncture; the CSF was otherwise normal. CSF analysis was done in 8 out of the 13 reported VrCVST cases, 3 of which were completely normal. Of the other 5 cases, 3 had mild pleocytosis and a mild increase in protein [19,23]; among these, only 1 confirmed the presence of the virus in the CSF by PCR. Two cases had a slight increase in protein in the CSF [16,20], with positive IgM in CSF in only 1 of them. These results indicate that VrCVST can occur as a complication of varicella, even without central nervous system (CNS) infection.
Most of the reported VrCVST cases excluded other causes of thrombophilia, which suggests that primary varicella can directly cause CVST. Protein S deficiency was reported in 3 of the 13 cases, of which 1 presented with protein C deficiency. Protein S and C deficiencies can be preexisting or induced by VZV infection [18,22]. VZV infection-induced protein S deficiency is reported in the literature to occur by inducing the production of anti-protein S [26]. Furthermore, transient and persistent emergence of the antiphospholipid antibodies were also reported in a few patients with VZV to induce arterial and/ or venous thrombosis [27,28]. Our patient had transient positive anticardiolipin and lupus anticoagulant, which could have caused the thrombosis. A recent systematic review of case reports found that particular infections can induce transient and persistent positive lupus anticoagulant and/or APS antibodies; many of these cases developed thrombosis [29]. However, the relationship between infection-induced transient positive APS antibodies and thrombosis needs further investigations to determine the significance and thus help determine strategies for prevention and treatment.
The pathogenesis of the thrombotic complications in VZV is unclear. However, it seems that different thrombotic manifestations are secondary to different etiologies and mechanistic pathways. Vasculopathic changes seem to be the main etiology for various manifestations, such as ischemic and hemorrhagic strokes, giant cell arteritis, external vasculopathy, and cranial neuropathies, among others, particularly in herpes zoster [30]. Two of the 11 VrCVST cases developed thrombosis in sites other than the CNS, which indicates a varicella-induced general prothrombotic status rather than CNS viral invasion-induced thrombosis. The former can be due to the direct activation of the coagulation cascade by the VZV infection or due to varicella-induced APS or protein S/C deficiency [18,22,31]. Normal CSF chemistry and negative VZV PCR in most of the reported cases further support this notion. No tissue histology was obtained to confirm these assumptions. Other VZV-related thrombotic compilations were attributed to vasculopathy due to direct infection of the blood vessels. This was confirmed by the presence of VZV DNA or its antibodies in the CSF in autopsy studies of patients who died of intracranial VZV-related vasculopathy [32]. It is important to note that most of the infection-related CVSTs were found to be due to local infections such as ears, sinuses, mouth, face, or neck rather than systemic infection, which might support the argument for viral invasion as a possible cause of VrCVST [9].
Furthermore, we found that all the reported VrCVT cases came from the Indian subcontinent, but it is unclear why this is so. However, we hypothesized possible explanations. First, it could be related to the low socioeconomic status and poor nutrition in the subcontinent. Second, it could be related to the unavailability of the vaccine in this population. Third, there might be genetic susceptibility of these patients to VZV, which makes them react to the virus differently, inducing vasculopathy and/or thrombosis. Fourth, the VZV genotype varies according to geographic distribution, and it could be that the VZV genotype that is prevalent in these countries is particularly pathogenic [33]. However, no data about the VZV genotype that infected these patients are available. Furthermore, our patient acquired the infection in Qatar, which is a part of the Middle East. All these hypotheses need further studies to establish correlations.
VrCVST usually has a favorable outcome. Most of the patients in the cases reported had improvement of their symptoms with no recurrence of seizure or thrombosis (Table 2). Most patients were treated with acyclovir, either IV or oral. For anticoagulation, heparin and vitamin K antagonist were mainly used. One patient was treated with apixaban, but he developed a pulmonary embolism and was thus switched to enoxaparin. One patient was treated successfully with dabigatran.
It is important to point out that the early recognition and the prompt treatment of CVST is crucial, as this was found to decrease the complications and significantly improve the outcomes [6]. Cerebral hernia caused by cerebral edema is the most common cause of death in patients with CVST. Although there were no deaths among the reported VrCVST patients, it is crucial to recognize malignant CVST when patients exhibit progressive symptoms, as these patients should be monitored closely and decompressive surgery should be promptly considered [34].
Conclusions
This report shows that primary VSV infection can be associated with vasculopathy and cerebral venous sinus thrombosis. Early recognition of this complication of VZV infection and prompt treatment are essential to prevent catastrophic complications.
Figures
References:
1.. Arvin AM, Varicella-zoster virus: Clin Microbiol Rev, 1996; 9(3); 361-81
2.. Guess HA, Broughton DD, Melton LJ, Kurland LT, Chickenpox hospitalizations among residents of Olmsted County, Minnesota, 1962 through 1981. A population-based study: Am J Dis Child, 1984; 138(11); 1055-57
3.. Preblud SR, Age-specific risks of varicella complications: Pediatrics, 1981; 68(1); 14-17
4.. Gnann JW, Varicella-zoster virus: Atypical presentations and unusual complications: J Infect Dis, 2002; 186(Suppl.1); S91-S98
5.. Ferro JM, Aguiar de Sousa D, Cerebral venous thrombosis: An update: Curr Neurol Neurosci Rep, 2019; 19(10); 74
6.. Luo Y, Tian X, Wang X, Diagnosis and treatment of cerebral venous thrombosis: A review: Front Aging Neurosci, 2018; 10; 2
7.. Coutinho JM, Ferro JM, Canhão P, Cerebral venous and sinus thrombosis in women: Stroke, 2009; 40(7); 2356-61
8.. Saposnik G, Barinagarrementeria F, Brown RD, Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2011; 42(4); 1158-92
9.. Duman T, Uluduz D, Midi I, A multicenter study of 1144 patients with cerebral venous thrombosis: The VENOST study: J Stroke Cerebrovasc Dis, 2017; 26(8); 1848-57
10.. Khealani BA, Wasay M, Saadah M, Cerebral venous thrombosis: A descriptive multicenter study of patients in Pakistan and Middle East: Stroke, 2008; 39(10); 2707-11
11.. Dentali F, Poli D, Scoditti U, Long-term outcomes of patients with cerebral vein thrombosis: A multicenter study [published correction appears in J Thromb Haemost. 2013;11(2):399]: J Thromb Haemost, 2012; 10(7); 1297-302
12.. Ruiz-Sandoval JL, Chiquete E, Bañuelos-Becerra LJ, Cerebral venous thrombosis in a Mexican multicenter registry of acute cerebrovascular disease: The RENAMEVASC study: J Stroke Cerebrovasc Dis, 2012; 21(5); 395-400
13.. Mathew T, Lobo A, Sarma GK, Nadig R, A case of post varicella cortical venous thrombosis successfully treated with dabigatran: Neurology India, 2013; 61(5); 531-32
14.. Menon B, Goyal R, Cerebral venous thrombosis as a complication of chicken pox: Ann Trop Med Public Heal, 2012; 5; 520
15.. Khan R, Yasmeen A, Pandey AK, Cerebral venous thrombosis and acute pulmonary embolism following varicella infection: Eur J Case Rep Intern Med, 2019; 6(10); 001171
16.. Mehta A, Arora A, Sharma M, Hemorrhagic stroke and cerebral venous thrombosis: rare neurological sequelae of chickenpox infection: Ann Indian Acad Neurol, 2018; 21(3); 228-32
17.. Imam SF, Lodhi OUH, Fatima Z, A unique case of acute cerebral venous sinus thrombosis secondary to primary varicella zoster virus infection: Cureus, 2017; 9(9); e1693
18.. Paul G, Paul BS, Singh G, Unseen face of varicella-zoster infection in adults: Indian J Crit Care Med, 2016; 20(12); 731-34
19.. Gayathri K, Ramalingam PK, Santhakumar R, Cerebral sinus venous thrombosis as a rare complication of primary varicella zoster virus infection: J Assoc Physicians India, 2016; 64(7); 74-76
20.. Sardana V, Mittal LC, Meena SR, Acute venous sinus thrombosis after chickenpox infection: J Assoc Physicians India, 2014; 62(8); 741-43
21.. Sada S, Kammineni A, Kanikannan MA, Afshan J, Cerebral sinus venous thrombosis: A rare complication of primary varicella zoster virus: Neurol India, 2012; 60(6); 645-46
22.. Siddiqi SA, Nishat S, Kanwar D, Cerebral venous sinus thrombosis: association with primary varicella zoster virus infection: J Stroke Cerebrovasc Dis, 2012; 21(8); 917 e1–4
23.. Shrivastava K, Sundaresan N, Senthilkumar P, Cerebral venous thrombosis following varicella infection: J Assoc Physicians India, 2016; 64(7); 68-69
24.. Sparaco M, Feleppa M, Bigal ME, Cerebral venous thrombosis and headache – a case-series: Headache, 2015; 55(6); 806-14
25.. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM, Cerebral venous thrombosis: Nat Rev Neurol, 2017; 13(9); 555-65
26.. Peyvandi F, Faioni E, Alessandro Moroni G, Autoimmune protein S deficiency and deep vein thrombosis after chickenpox: Thromb Haemost, 1996; 75(1); 212-13
27.. Frontino G, Passoni A, Piscopo MA, Bilateral cavo-ilio-femoral thrombosis in an adolescent with transient anti-phospholipid antibodies and factor V heterozygous mutation: A case report: Cases J, 2009; 2; 6830
28.. Uthman IW, Gharavi AE, Viral infections and antiphospholipid antibodies: Semin Arthritis Rheum, 2002; 31(4); 256-63
29.. Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME, Systematic review of case reports of antiphospholipid syndrome following infection: Lupus, 2016; 25(14); 1520-31
30.. Nagel MA, Gilden D, Update on varicella zoster virus vasculopathy: Curr Infect Dis Rep, 2014; 16(6); 407
31.. Mandel EE, Hypercoagulability: Chic Med, 1961; 63; 19-24
32.. Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5 – 1995 revisited: Neurology, 1996; 47(6); 1441-46
33.. Hosogai M, Nakatani Y, Mimura K, Genetic analysis of varicella-zoster virus in the aqueous humor in uveitis with severe hyphema: BMC Infect Dis, 2017; 17; 427
34.. Théaudin M, Crassard I, Bresson D, Should decompressive surgery be performed in malignant cerebral venous thrombosis? A series of 12 patients: Stroke, 2010; 41(4); 727-31
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250