05 April 2021: Articles 
Nerve Sheath Myxoma in the Lower Extremity: A Rare Case with Description of Magnetic Resonance Imaging and Sonographic Findings
Rare disease
Dawood A. Tafti1ABCDEFG*, Micheal C. Dearborn2BDEF, Ashley Ornoff3BCDEF, Adam R. Moeck2E, Nathan D. Cecava245ABCDEFDOI: 10.12659/AJCR.927922
Am J Case Rep 2021; 22:e927922
Abstract
BACKGROUND: This report is of a nerve sheath myxoma presenting as a slow-growing mass in the back of the left ankle of a 36-year-old man that was investigated by ultrasound and magnetic resonance imaging (MRI) before the diagnosis was confirmed by histopathology.
CASE REPORT: We report a nerve sheath myxoma of the ankle in a 36-year-old man. The palpable abnormality was falsely assumed to be a ganglion cyst prior to advanced imaging. Magnetic resonance imaging demonstrated a lobular mass with high T2 and intermediate T1 signal as well as moderate enhancement. T2 sequences also demonstrated distinctive internal septae. These internal septae were also noted on sonographic evaluation prior to biopsy. The patient was treated with surgical excision, and pathologic analysis showed myxoid nodules with loose arrangements of spindled cells separated by fibrous septae. S-100 protein and glial fibrillary acidic protein positivity by immunohistochemistry staining was demonstrated. Follow-up imaging at 12 months showed no evidence of tumor recurrence.
CONCLUSIONS: This case highlights that while nerve sheath myxomas are rare tumors, they should be considered in cases of cutaneous soft-tissue masses with myxoid imaging features. Ultrasound and magnetic resonance imaging features of thin internal septae may be present and correspond well with the unique histopathological characteristics of these lesions. This report shows the importance of imaging of peripheral soft-tissue masses, including ultrasound and MRI, which can identify localized and benign features and the solid, cystic, and myxoid areas, which were characteristic in this case of benign nerve sheath myxoma.
Keywords: Nerve Sheath Neoplasms, Neurothekeoma, Peripheral Nervous System Neoplasms, Immunohistochemistry, Lower Extremity, Magnetic Resonance Imaging, Myxoma
Background
First described in 1969 by Harkin and Reed, neurothekeomas and nerve sheath myxomas (NSM) are rare cutaneous neoplasms of peripheral nerve origin and are characterized as benign soft-tissue tumors according to current guidelines [1–3]. In 2020, the World Health Organization (WHO) classified nerve sheath myxomas as one of 12 subtypes of soft-tissue tumors [3]. Although previously used interchangeably, neurothekeomas and NSMs are now described as unique entities, with the later exhibiting S100-positivity [4,5]. In addition to diffuse immunoreactivity to S100, NSMs also demonstrate moderate to diffuse reactivity to GFAP and CD57 and show strong immunoreactivity to collage IV around tumor cells [3]. One case series demonstrates that NSMs show a preponderance in females and the mean age of incidence is 36 years [5,6]. NSMs most commonly occur in the extremities and often present as small flesh-colored papules and tend to be slow-growing [5,7–12].
Surgical excision is usually the treatment of choice in symptomatic patients, with a high recurrence rate in cases of incomplete excision [3,13]. We present a case report of a NSM occurring in the subcutis of the ankle, a somewhat rare location for these tumors. We also describe the sonographic and magnetic resonance imaging (MRI) features of this tumor. To our knowledge there have been only 4 other reported cases of a NSM at the ankle, and no case reports have published ultra-sound images [5]. Ultrasound and MRI provide excellent anatomic detail in the characterization of soft-tissue tumors, and identifying the unique imaging features of NSMs can help in their diagnosis when surgical excision is not an option [14]. Radiography can also add information regarding matrix calcification and osseous involvement [15]. Benign neoplasms constitute the majority of foot and ankle masses, and imaging shows that benignity includes well-defined margins and contiguity with adjacent nerves [16,17]. The purpose of this report is to highlight the clinical presentation, imaging appearance, and histopathology of these rare tumors. This report is of a nerve sheath myxoma presenting as a slow-growing mass in the back of the left ankle of a 36-year-old man that was investigated by ultrasound and magnetic resonance imaging (MRI) before the diagnosis was confirmed by histopathology.
Case Report
A 36-year-old man presented to an outpatient primary care clinic for evaluation of a left posterolateral ankle palpable mass. Past medical history was notable for shin splints. The patient had no history of prescription medication and he used over-the-counter naproxen occasionally for lower-extremity pain. The patient denied a current history of tobacco, alcohol, or drug use. The patient reported that the ankle mass had existed for the past 14 years and was previously diagnosed as a ganglion cyst. The mass was treated conservatively until it started to become symptomatic, exhibiting “stinging” pain, pressure, and gradual increase in size for 8 months prior to the patient’s presentation. Physical exam showed a firm, palpable, flesh-colored nodule measuring 1×1 cm located at the posterolateral ankle, distal to the lateral malleolus. There were no skin discolorations or neuromuscular deficits.
The patient was prescribed naproxen, 500 mg by mouth, twice daily for the pain associated with the lesion. An initial radio-graph performed of the ankle demonstrated a nonspecific soft-tissue density posterior to the talus, only visible on the lateral radiograph (Figure 1). On MRI, the lesion appeared as a lobular mass within the posterolateral subcutaneous fat and contiguous with the dermis (Figure 2). The mass showed bright T2 and intermediate T1 signal with moderate enhancement following intravenous gadolinium contrast administration. On T2 fat-suppressed images, the mass more distinctively showed internal thin, dark signal septae. The mass measured 1.2×1.3×1.4 cm (anteroposterior×transverse×craniocaudal) in size.
Given the nonspecific imaging features of the mass and inability to exclude soft-tissue sarcoma, the patient was referred to orthopedic oncology and then to the musculoskeletal radiology service for ultrasound-guided biopsy. Pre-biopsy sono-graphic evaluation of the mass showed a well-circumscribed, hypoechoic mass without internal vascularity and with posterior sonographic enhancement (Figure 3). Corresponding to the MRI findings, there were scattered isoechoic internal septae within the mass. Ultrasound-guided biopsy was performed with a 14-gauge spring-loaded needle device.
The patient was scheduled for a surgical excision, which was performed without complications. The excisional specimen consisted of a yellow-tan, fibrofatty, firm mass measuring 2×1.9×2 cm. Serial sectioning revealed a white-tan to yellow-tan, rubbery cut surface with multiple lobulations. Sections of the initial biopsy and excisional specimens showed large, irregular, myxoid nodules containing loose arrangements of spindled cells with small nuclei and few scattered bland fibrohistiocytoid cells. The nodules were separated by internal thick fibrous bands (Figure 4). No high-grade features, such as nuclear enlargement, hyperchromasia, nuclear membrane irregularities, or mitotic figures, were identified. The tumor displayed diffuse and strong staining for S-100 protein and glial fibrillary acidic protein (GFAP) by immunohistochemistry (Figure 5). The histology and staining patterns were confirmatory for a nerve sheath origin tumor and were consistent with a dermal NSM.
One month following resection, the patient stated that his symptoms had significantly improved, with mild occasional numbness and “tingling” along the left lateral malleolus. Follow-up MRI 6 months after resection demonstrated residual nonmasslike T2 hyperintense signal and mild non-masslike enhancement in the surgical bed. Follow-up MRI at 12 months demonstrated resolution of T2 signal abnormality and no enhancement consistent with absent tumor recurrence (Figure 6). No treatment complications were recorded on follow-up clinic visits.
Discussion
NSMs are rare, benign nerve sheath tumors that primarily occur in superficial locations [5]. Although usually asymptomatic, pain can be a presenting symptom [5]. Until recently, the terms NSM and neurothekeoma have been used interchangeably; however, these tumors are now differentiated by immunohistochemistry with NSMs exhibiting S100-positivity [4,5]. Reported NSMs are more common in the upper extremities, especially the hands. When occurring in the lower extremities, the knee and foot are the most common locations [5,8,9]. To our knowledge, there have been only 4 other reported cases of a NSM at the ankle [5].
In 2005, Fetsch et al published the largest clinicopathologic NSM case series, with 57 cases [5]; imaging features of NSMs were not reported, 86% of their cases occurred in the extremities, and most lesions were present for many years prior to surgical resection. They had follow-up data for 34 cases and 47% of these had local recurrence, highlighting the importance of performing a resection with margins clear of disease. Histology features of these lesions common to our case include multinodular/multilobular masses in dermal and/or subcutis locations with abundant myxoid matrix and a peripheral fibrous border. Correlating with our findings of internal septae on imaging, their specimens showed fibrous connective tissue bordering the multinodular tumor architecture. Dominant cellular tumor components were epithelioid Schwann cells in corded, nested, and/or syncytial-like aggregates [5].
Fetsch et al were the first to suggest that NSMs are a subset of peripheral nerve sheath tumors and are distinct from neurothekeomas based on the latter lacking S-100 and GFAP staining and a fibrous border. Neurothekeomas also have less myxoid matrix, more cellular spindling, and more nuclear variability than NSMs [5]. Pathologic differential diagnosis for NSM includes neurothekeoma, superficial angiomyxoma, neurofibroma, schwannoma, perineurial tumors, chondroma of soft parts, and superficial acral fibromyxoma [5].
Sonographic and MRI features of NSMs have been infrequently described in the literature, especially in cases occurring in the extremities. This is likely due to the typical dermal and subcutis locations of these lesions, with physicians opting for surgical excision without imaging. The imaging features for many soft-tissue masses are very nonspecific as most benign soft-tissue masses cannot reliably be distinguished from malignant sarcomas or metastatic disease on imaging. Most soft-tissue cases require histopathologic evaluation; however, consideration of the imaging features remains important for assessing lesion imaging and pathologic correlation.
This case of NSM highlights some important imaging features reflected in the histopathology of these lesions. First, the background T2 signal of these lesions are very bright, in keeping with the myxoid components (extracellular mucin). Lesions with myxoid components sharing this bright T2 MRI signal characteristic include benign lesions such as myxomas and peripheral nerve sheath tumors [21–27], but also include malignant lesions such as myxoid liposarcomas and chondrosarcomas, among others. Similar to NSMs, benign myxomatous lesions show more homogeneous T2 bright myxoid signal whereas malignant myxomatous lesions tend to have more heterogeneous components and are likely to show features related to their cell of origin [28].
Bright T2 myxoid-like signal shown in the present case report is consistent with MRI features described in prior NSM case reports in the upper extremities, orbital, and intracranial locations (Table 1) [18,19,30–32]. Myxoid components with high water content also promote ultrasound features of hypoechogenicity and posterior acoustic enhancement. This case report is the only known case report in the literature to show NSM ultrasound figures. A recent case report in the hand reported ultrasound features of a lobulated, circumscribed, and hyper-echoic mass but did not publish the images [30].
The current authors believe that internal dark signal septae shown in this case report are an important feature which could be characteristic of NSMs, and this finding should be evaluated in future NSM studies. Internal septae are most evident on T2-weighted and post-contrast sequences. On ultrasound, septae may be isoechoic on a hypoechoic lesion background, as in this case report. Internal septae seen at imaging correlate with the fibrous septae seen on NSM histopathology. Including this case report, dark signal septations were present in 3 of 7 NSMs case reports with MR imaging (Table 1). Older case reports might have not been able to demonstrate internal septations as current MRI techniques allow for better tissue contrast and spatial resolution. Understanding the imaging characteristics of soft-tissue lesions of the ankle and foot are important considering MRI plays an important role in the management of these tumors when greater than 2 cm. A thorough physical exam and imaging features help differentiate between determinate and indeterminate lesions, which subsequently guides biopsy and surgical excision [33].
Conclusions
A case of benign nerve sheath myxoma in the back of the left ankle of a 36-year-old man was presented. This report has shown the importance of imaging of peripheral soft-tissue masses, including ultrasound and MRI, which can identify localized and benign features and the solid, cystic, and myxoid areas, which were characteristic in this case of benign nerve sheath myxoma. Nerve sheath myxomas are a rare soft-tissue mass most often encountered in the dermis of the upper extremities or to a lesser extent in the lower extremities. Specimen staining with S100 positivity can differentiate these tumors from previously associated neurothekeomas. This case report describes a rare case in the ankle, with discussion of infrequently reported ultrasound and MRI features that correlate to histopathological findings in these tumors. When a dermal-based, solid mass is encountered with imaging showing bright T2 myxoid signal and internal septae, nerve sheath myxoma should be considered in the differential list of possibilities.
Figures
References:
1.. Harkin JC, Reed RJ: Tumors of the peripheral nervous system Atlas of Tumor Pathology Second Series, Fascicle 3, 1969; 60-64, Washington DC, Armed Forces Institute of Pathology
2.. von Mehren M, Randall RL, Benjamin RS, Soft tissue sarcoma, Version 2.2018, NCCN clinical practice guidelines in oncology: J Natl Compr Canc Netw, 2018; 16(5); 536-63
3.. : WHO classification of tumours. soft tissue and bone tumours, 2020, Lyon, France, IARC Press
4.. Sheth S, Li X, Binder S, Differential gene expression profiles of neurothekeomas and nerve sheath myxomas by microarray analysis: Mod Pathol, 2011; 24(3); 343-54
5.. Fetsch JF, Laskin WB, Miettinen M, Nerve sheath myxoma: A clinicopatho-logic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate: Am J Surg Pathol, 2005; 29(12); 1615-24
6.. Carvajal JA, Cuartas E, Qadir R, Peripheral nerve sheath tumors of the foot and ankle: Foot Ankle Int, 2011; 32(2); 163-67
7.. Spadari F, Guzzi G, Bombeccari GP, Nerve sheath myxoma of the tongue: Acta Dermatovenerol Croat, 2014; 22(1); 52-56
8.. Persich G, Portela M, Neurothekeoma in the foot: A rare occurrence: J Am Podiatr Med Assoc, 2004; 94(1); 59-60
9.. Gehrke JC, Hamson KR, Havey AD, Dermal nerve sheath myxoma of the hallux: A case report: Foot Ankle Int, 1994; 15(12); 666-68
10.. Suh YL, Song KY, Kim JM, Nerve sheath myxoma (neurothekeoma) – a case report: J Korean Med Sci, 1992; 7(1); 85-89
11.. Bergamin F, Gangemi EN, Cerato C, An unusual case of neurothekeoma of the arm in an adult: J Orthop Traumatol, 2016; 17(3); 287-90
12.. Bhat A, Narasimha A, Nerve sheath myxoma: Report of a rare case: J Clin Diagn Res, 2015; 9(4); ED07-9
13.. Sage RJ, Lopiccolo MC, Laungani AG, Mohs micrographic surgery for the treatment of cellular neurothekeoma and review of its use in surgical management of benign tumors: Dermatol Surg, 2010; 36(7); 1214-18
14.. Hughes P, Miranda R, Doyle AJ, MRI imaging of soft tissue tumours of the foot and ankle: Insights Imaging, 2019; 10(1); 60
15.. Noebauer-Huhmann IM, Weber MA, Lalam RK, Soft tissue tumors in adults: ESSR – approved guidelines for diagnostic imaging: Semin Musculoskelet Radiol, 2015; 19; 475-82
16.. Woertler K, Soft tissue masses in the foot and ankle: Characteristics on MR Imaging: Semin Musculoskelet Radiol, 2005; 9(3); 227-42
17.. Li CS, Huang GS, Wu HD, Differentiation of soft tissue benign and malignant peripheral nerve sheath tumors with magnetic resonance imaging: Clin Imaging, 2008; 32(2); 121-27
18.. O’rourke H, Meyers SP, Katzman PJ, Neurothekeoma in the upper extremity: Magnetic resonance imaging and computed tomography findings: J Comput Assist Tomogr, 2005; 29(6); 847-50
19.. Vij M, Jaiswal S, Agrawal V, Nerve sheath myxoma (neurothekeoma) of cerebellopontine angle: Case report of a rare tumor with brief review of literature: Turk Neurosurg, 2013; 23(1); 113-16
20.. Kim HJ, Baek CH, Ko YH, Neurothekeoma of the tongue: CT, MR, and FDG PET imaging findings: AJNR Am J Neuroradiol, 2006; 27(9); 1823-25
21.. Kakkar C, Shetty CM, Koteshwara P, Telltale signs of peripheral neurogenic tumors on magnetic resonance imaging: Indian J Radiol Imaging, 2015; 25(4); 453-58
22.. Chee DW, Peh WC, Shek TW, Pictorial essay: Imaging of peripheral nerve sheath tumours: Can Assoc Radiol J, 2011; 62(3); 176-82
23.. Lin J, Martel W, Cross-sectional imaging of peripheral nerve sheath tumors: Characteristic signs on CT, MR imaging, and sonography: Am J Roentgenol, 2001; 176(1); 75-82
24.. Beaman FD, Kransdorf MJ, Menke DM, Schwannoma: Radiologic-pathologic correlation: Radiographics, 2004; 24(5); 1477-81
25.. Jee WH, Oh SN, Mccauley T, Extraaxial neurofibromas versus neurilemmomas: Discrimination with MRI: Am J Roentgenol, 2004; 183(3); 629-33
26.. Stull MA, Moser RP, Kransdorf MJ, Magnetic resonance appearance of peripheral nerve sheath tumors: Skeletal Radiol, 1991; 20(1); 9-14
27.. Wasa J, Nishida Y, Tsukushi S, MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas: Am J Roentgenol, 2010; 194(6); 1568-74
28.. Petscavage-Thomas J, Walker EA, Logie CI, Soft-tissue myxomatous lesions: Review of salient imaging features with pathologic comparison: Radiographics, 2014; 34(4); 964-80
29.. Malkoc M, Ormeci T, Keskinbora M, Nerve sheath myxoma of the dorsal paravertebral space: Int J Surg Case Rep, 2014; 5(11); 858-60
30.. Khashaba H, Hafez E, Burezq H, Nerve sheath myxoma: A rare tumor, a case report and literature review: Int J Surg Case Rep, 2020; 73; 183-86
31.. Bulduk EB, Aslan A, Öcal Ö, Neurothekeoma in the middle cranial fossa as a rare location: Case report and literature review: Neurochirurgie, 2016; 62(6); 336-38
32.. Sánchez-Orgaz M, Grabowska A, Arbizu-Duralde A, Orbital nerve sheath myxoma: A case report: Ophthalmic Plast Reconstr Surg, 2011; 27(4); e106-8
33.. DeGroot H, Approach to the management of soft tissue tumors of the foot and ankle: Foot Ankle Spec, 2008; 1(3); 168-76
Figures
Tables
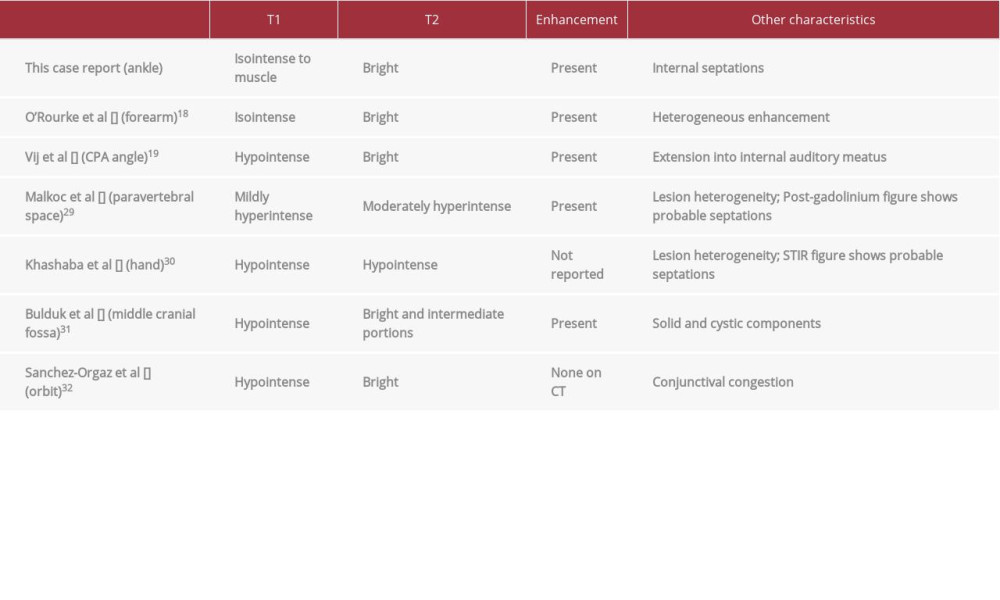 Table 1.. Table of case reports of nerve sheath myxomas with descriptions of magnetic resonance imaging characteristics. Features listed include T1 and T2 signal characteristics, enhancement, as well as other noteworthy features.
Table 1.. Table of case reports of nerve sheath myxomas with descriptions of magnetic resonance imaging characteristics. Features listed include T1 and T2 signal characteristics, enhancement, as well as other noteworthy features. Table 1.. Table of case reports of nerve sheath myxomas with descriptions of magnetic resonance imaging characteristics. Features listed include T1 and T2 signal characteristics, enhancement, as well as other noteworthy features.
Table 1.. Table of case reports of nerve sheath myxomas with descriptions of magnetic resonance imaging characteristics. Features listed include T1 and T2 signal characteristics, enhancement, as well as other noteworthy features. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








