10 April 2021: Articles 
Hodgkin Lymphoma-Associated Superior Vena Cava Syndrome: A Case Report and Review of the Literature
Rare coexistence of disease or pathology
Ruoning Ni1ABDEF*, Mahmoud Amr1ABCDEFG, Abhishek Kalla2ABCDEFGDOI: 10.12659/AJCR.929437
Am J Case Rep 2021; 22:e929437
Abstract
BACKGROUND: Hodgkin lymphoma (HL) is a relatively rare etiology of superior vena cava (SVC) syndrome, with only 24 cases reported in the literature. The characteristics, management, and prognosis of HL-associated SVC syndrome remain unclear. This case report describes nodular sclerosis classical HL and the associated clinical manifestations presenting as SVC syndrome in a middle-aged patient, and it summarizes the characteristics of HL-associated SVC syndrome.
CASE REPORT: In this case report, we present a 53-year-old Hispanic man with progressively worsening dyspnea, dry cough, facial and neck edema, and dysphagia. SVC syndrome was diagnosed, and pathology revealed nodular sclerosis classical HL. The patient was treated with doxorubicin, bleomycin, vinblastine, and dacarbazine. SVC syndrome improved, and repeated imaging showed that the lymphoma had decreased in size and had become metabolically inactive.
CONCLUSIONS: We reviewed the characteristics, management, and prognosis of HL-associated SVC syndrome, which may indicate more advanced and recurrent progression in patients with HL. This possibility suggests that physicians should provide urgent diagnosis and closer follow-up, and more aggressive therapies may be needed because of the high risk of recurrence. Therapy may induce late-onset SVC syndrome in patients with HL.
Keywords: Hodgkin Disease, review, Superior Vena Cava Syndrome, Vena Cava, Superior
Background
Superior vena cava (SVC) syndrome was first described in 1757 by the Scottish surgeon Dr. William Hunter in relation to a patient with syphilitic aortitis [1]. SVC syndrome, also known as superior mediastinal syndrome, is a condition caused by any compression or obstruction of the SVC, including direct invasion by a malignancy [2]; external compression by tumors, aortic aneurysm, or fibrosis [3]; or thrombosis secondary to indwelling central venous catheters or leads from defibrillators and pacemakers [2]. In the 20th century, malignancy accounted for approximately 60% to 85% of SVC syndrome cases [2,4,5]. Among all cases, non-small-cell lung cancer, small cell lung cancer, and non-Hodgkin lymphoma are estimated to be responsible for about 95% of the cases of malignancy-induced SVC syndrome [2].
However, the relationship between Hodgkin lymphoma (HL) and SVC syndrome is not well-established. HL remains uncommon, and there are an estimated 8110 cases per year in the United States, accounting for about 0.6% of all cancers and 10% of all lymphomas [6]. HL can be divided into 2 major groups, classical HL and nodular lymphocyte predominant HL, from morphological and immunophenotypical perspectives. Classical HL accounts for approximately 90% of HL [7], of which the nodular sclerosis classical HL (NSCHL) subtype accounts for 70% [8]. Peak incidence of NSCHL occurs between 15 and 35 years old, with male preponderance [9], and it varies with race [10]. Given the limited number of individuals diagnosed with HL, the occurrence of SVC syndrome is even rarer, with only 24 cases reported in the literature.
The objective of this case report was to describe NSCHL in a middle-aged patient presenting with SVC syndrome and associated clinical manifestations. This case also reviews other previous reports of HL with SVC syndrome and overall prognosis and management.
Case Report
A 53-year-old Hispanic man with no significant past medical history presented to the Emergency Department with multiple concerns.
The patient had been in his usual state of health until 3 weeks prior to admission. He noticed progressively worsening dyspnea and nonproductive cough. The symptoms improved when he leaned to his left side and worsened when he leaned to the right. He also reported having dull right substernal chest pain at rest, radiating to his throat and right back, not associated with food intake, exercise, or temperature change. He mentioned having bothersome nocturnal diaphoresis with associated subjective fevers, chills, and rigors. He complained of dysphagia starting 2 weeks prior to admission, initially to solid foods and eventually even thick liquids. He started to notice facial and neck edema 2 days prior to presentation with associated plethora, which extended into the right upper extremity, subjectively. Additionally, he also described blurry vision and generalized constant headache. He denied any fatigue, syncope, orthopnea, paroxysmal night dyspnea, hoarseness, or dysphonia.
The patient had no significant medical history or any surgical history. His only medication was acetaminophen, which he used as needed. The patient stopped social cigarette smoking more than 30 years earlier and denied regular alcohol use or any illicit drug abuse. He was originally from Mexico and used to work as a telephone pole installer. At the time of presentation, he was working as a painter and construction worker. He denied any recent travel or sick contact. He lived with his family and was monogamous. His family history was un-remarkable other than his mother who died of gastric cancer.
He visited his primary care physician for the above symptoms and was prescribed amoxicillin, azithromycin, and amoxicillin/clavulanate. There was no improvement over the following 2 weeks. In the Emergency Department his temperature was 37.5°C, the heart rate 111 beats/min, blood pressure 153/101 mmHg, respiratory rate 20 breaths/min, and oxygen saturation 96% on room air. He had mild respiratory distress upon presentation and dry mucosa. He had facial, neck, and upper thorax plethora, jugular venous distention, and scleral injection. He did not have any periorbital edema, chest congestion, or dilated thoracic veins. Pemberton’s sign was negative. He had diminished but equal breath sounds bilaterally without stridor, crackles, wheezing, or rhonchi. He was tachycardic, but he did not have a cardiac murmur or gallop. He also had no hepatomegaly or splenomegaly, and no palpable superficial lymphadenopathy. The remainder of the physical examination was unremarkable.
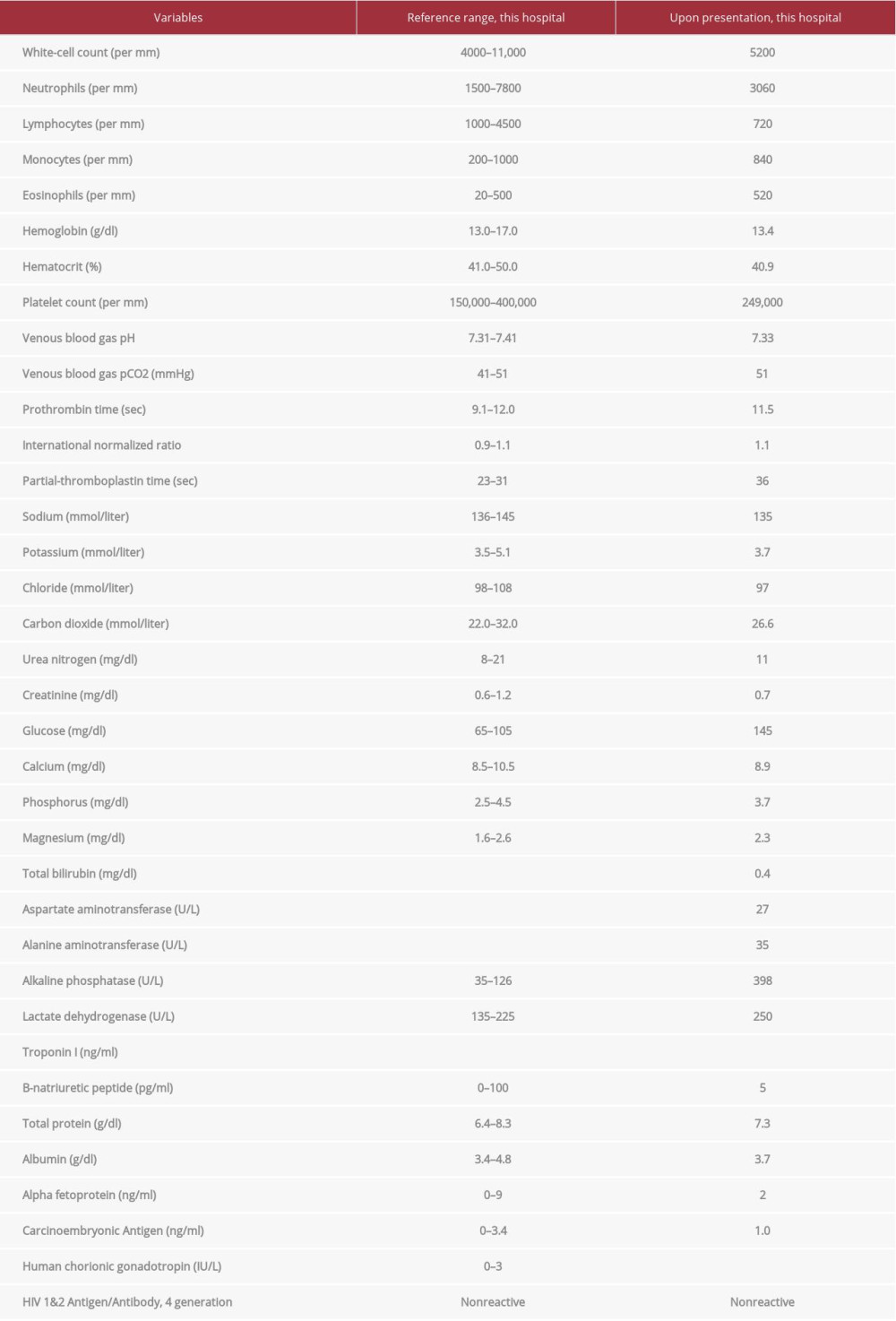
Laboratory testing revealed a white blood cell count of 5200/μL, lymphocyte count 720/μL (14.0%), eosinophils 520/μL (10.1%), albumin 3.7 g/dL, lactate dehydrogenase 250 U/L, and alkaline phosphatase 398 U/L. An HIV antigen/antibody test was negative, and other blood tests showed normal ranges for hemoglobin, platelets, venous blood gas, creatinine, calcium, phosphorus, aspartate aminotransferase, alanine aminotransferase, bilirubin, troponin I, B-natriuretic peptide, alpha-fetoprotein, carcinoembryonic antigen, and human chorionic gonadotropin. The laboratory test results are shown in Table 1. An electrocardiogram showed the sinus rhythm with no ST-T change.
A frontal and lateral radiography of the chest showed a large mediastinal opacity. Computed tomography (CT) of the neck and chest revealed diffuse conglomerated adenopathy in the right paratracheal region, with the largest mass measuring up to 8.4×7.3×8.3 cm causing significant compression of the right upper lobe lobar pulmonary artery and the SVC, as well as bilateral pleural effusion. There was no evidence of pulmonary embolism. There were a few prominent lymph nodes in the lower neck (levels 5B, 6, and 7) at the thyroid level, measuring up to 1.2 cm in the short axis (axial 58), and posterior to SVC (axial 154) (Figure 1).
CT of the abdomen and pelvis with intravenous contrast revealed multiple ill-defined round and elongated areas of lucency within the liver and splenic compatible with metastatic disease. Lymphadenopathy was identified in the periportal, periaortic retroperitoneal, celiac, and gastrohepatic regions. While undergoing workup for suspected lymphoma based on the CT finding, the patient was started on dexamethasone 4 mg twice daily to help relieve his SVC symptoms. Endobronchial ultrasound-guided bronchoscopy and transbronchial needle aspirate of the right paratracheal lymph node at station 7 was performed on day 3, but no immunophenotypic or morphologic abnormalities were identified. The sample was also negative for acid-fast bacilli or fungus stain. Due to a strong suspicion of malignancy, the multidisciplinary thoracic tumor board discussion led to a video-assisted thoracoscopy. The thoracos-copy and robotic assisted excisional biopsy of the mediastinal mass were performed on day 10. Findings included a few Reed-Sternberg cells, which were CD30+ and weakly CD15+, with scattered small CD20+ B cells, which were compatible with NSCHL (Figure 2A, 2B). By the time of the patient’s discharge, 5 days after starting dexamethasone, his SVC presentation had significantly improved, with reduced facial, neck, and arm swelling.
Outpatient positron emission tomography (PET)/CT demonstrated metabolically active lymphadenopathy involving the neck, chest, and abdomen, with a maximum standardized uptake value (SUV) of 15.6, as well as metabolic evidence of extranodal lymphomatous involvement of the liver, spleen, and the visualized axial skeleton, including the sternum, vertebrae, sacrum, and ischium (Figure 3A). As per the National Comprehensive Cancer Network (NCCN) guidelines, the patient was treated with doxorubicin (Adriamycin), bleomycin, vinblastine, and dacarbazine (ABVD regimen) for 2 cycles and then restaged with a PET/CT, which revealed minimal remaining fluoro-deoxyglucose (FDG) uptake (Deauville 2). Hence, he was subsequently treated with AVD (bleomycin omitted) for an additional 4 cycles, to complete 6 cycles total of antineoplastic therapy. Follow-up FDG PET/CT demonstrated a metabolically inactive (SUV=2.73) right paratracheal conglomerate, resolution or decrease in size of the bilateral non-FDG avid nodes, and no metabolic evidence of new lymphadenopathy or distal metastasis into the liver, spleen, or bones (Figure 3B). The patient has since been on an NCCN-based follow-up schedule and continues to be in remission.
Discussion
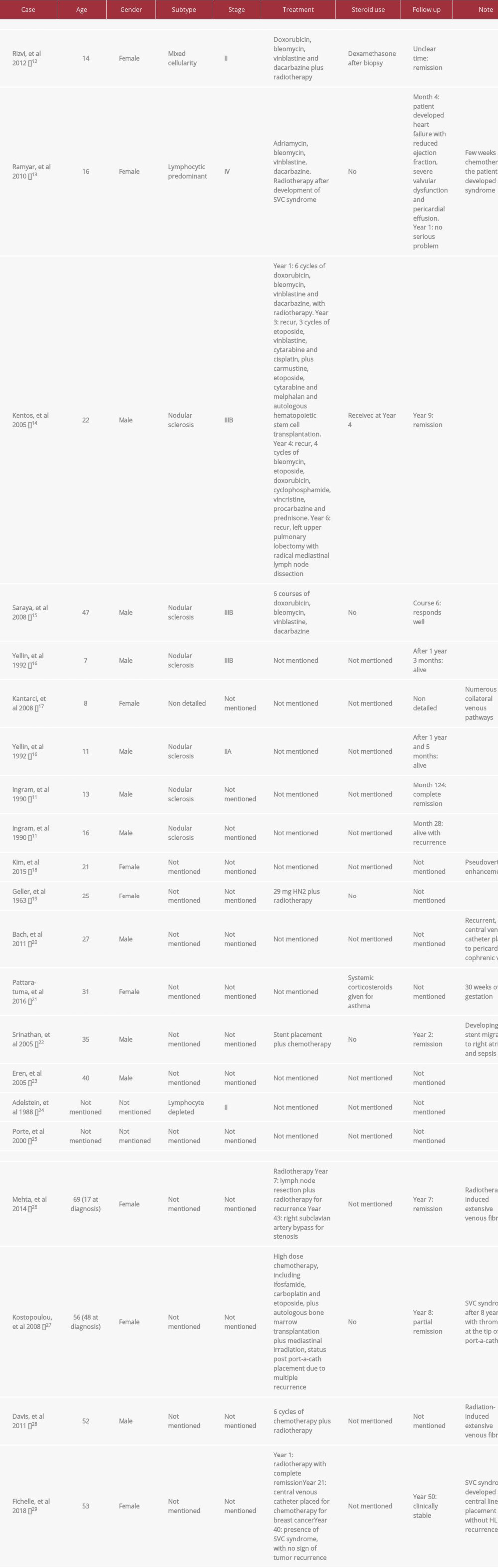
HL can present as a mediastinal mass, but the occurrence of SVC syndrome is much lower than other forms of lymphoma. In a study by Ingram et al [11], 31% of 333 pediatric patients with HL presented with a mediastinal mass, but the prevalence of SVC syndrome was as low as 2%. In the literature on adults, HL-associated SVC syndrome is rare, with only 24 cases reported, including 20 cases of HL that initially presented as SVC syndrome and 4 cases in which SVC syndrome developed under the influence of therapy [12–29] (Table 2).
Among the HL cases that initially presented as SVC syndrome, patients aged from 7 to 47 years old, predominantly around adolescence, with a sex ratio of approximately 1: 1. NSCHL was the majority subtype in the reported cases. When SVC syndrome was noted on initial presentation, the individuals had a more advanced stage at diagnosis. Recurrent HL may require combined chemotherapy, radiotherapy, autologous bone marrow transplantation, and in rare cases, surgery. Yellin et al [16] reported that, compared with non-Hodgkin lymphoma, clinical response and radiologic response to therapy are noted to be slower in HL that initially presents as SVC syndrome in children [16].
Our patient had an International Prognostic Score of 4 (male, age, hypoalbuminemia, stage IV), with an estimated rate of 51±4% of freedom from progression and overall survival rate of 61±4% [30]. The presence of male sex, age more than 45 years old, stage IV, and B symptoms also predicts 82% of the 5-year survival rate according to Gisselbrecht et al [31]. However, due to rare prevalence and limited data, predicting the prognosis of HL with an initial presentation of SVC syndrome remains difficult.
SVC syndrome may develop during or after therapy for HL. The iatrogenic complications include catheter-induced SVC thrombosis and radiation-induced venous fibrosis eventually resulting in obstruction of the SVC. Currently, widespread use of indwelling venous catheters for chemotherapy increases the risk of developing venous thrombosis. Hypercoagulability in HL may also contribute to late-onset SVC syndrome [27]. Additionally, radiation therapy has been frequently used in HL, especially recurrent HL. The proliferation of intimal cells, thickening of tunica intima, and loss of vasa vasorum secondary to radiation may all cause arterial and venous fibrosis after decades [26,28,29].
As an oncologic emergency, SVC syndrome may require immediate management guided by the grade of severity [32]. Endovenous recanalization, such as thrombolysis, thrombectomy, angioplasty, and SVC stenting, should be considered in the setting of respiratory compromise or depressed brain function. However, the stent placement in SVC to relieve mass compression in the emergent setting may be associated with complications, such as sepsis or stent migration after tumor response to chemotherapy [22]. The role of long-term anti-platelet and/or anticoagulation for SVC stent placement remains uncertain. Glucocorticoids may also be used to mitigate emergent obstruction as a therapeutic effect in HL, although they may obscure the biopsy results. In our patient, the initial use of dexamethasone may have contributed to the negative result of bronchoscopy biopsy. Head elevation, supplemental oxygen, or diuretics for symptomatic relief in some situations may be more reasonable before a biopsy has been obtained to avoid affecting diagnostic sensitivity.
Conclusions
HL remains a relatively rare cause of SVC syndrome. When initially presenting as SVC syndrome, it may indicate a more advanced phase of HL that requires urgent diagnosis and closer follow-up, and it may need more aggressive therapies due to the high possibility of recurrence. In addition, indwelling venous catheter, previous radiation, and hypercoagulability in HL may contribute to late-onset SVC syndrome.
Figures
References:
1.. Hunter W, Johnston W: The history of an aneurysm of the aorta, with some remarks on aneurysms in general, 1757; 1; 323-57, London, Medical observations and inquiries
2.. Rice TW, Rodriguez RM, Light RW, The superior vena cava syndrome: Clinical characteristics and evolving etiology: Medicine (Baltimore), 2006; 85(1); 37-42
3.. Loyd JE, Tillman BF, Atkinson JB, Mediastinal fibrosis complicating histoplasmosis: Medicine (Baltimore), 1988; 67(5); 295-310
4.. Yellin A, Rosen A, Reichert N, Superior vena cava syndrome. The myth – the facts: Am Rev Respir Dis, 1990; 141(5 Pt 1); 1114-18
5.. Schraufnagel DE, Hill R, Leech JA, Superior vena caval obstruction. Is it a medical emergency?: Am J Med, 1981; 70(6); 1169-74
6.. Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019: Cancer J Clin, 2019; 69(1); 7-34
7.. Laurent C, Do C, Gourraud PA, Prevalence of common non-Hodgkin lymphomas and subtypes of Hodgkin lymphoma by nodal site of involvement: A systematic retrospective review of 938 cases: Medicine (Baltimore), 2015; 94(25); e987
8.. Swerdlow SH, Campo E, Harris NL: WHO classification of tumours of haematopoietic and lymphoid tissues, 2017, Lyon, France, IARC
9.. Morton LM, Wang SS, Devesa SS, Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001: Blood, 2006; 107(1); 265-76
10.. Evens AM, Antillón M, Aschebrook-Kilfoy B, Racial disparities in Hodgkin’s lymphoma: A comprehensive population-based analysis: Ann Oncol, 2012; 23(8); 2128-37
11.. Ingram L, Rivera GK, Shapiro DN, Superior vena cava syndrome associated with childhood malignancy: Analysis of 24 cases: Med Pediatr Oncol, 1990; 18(6); 476-81
12.. Rizvi I, Zaman S, Zaidi N, Superior vena cava syndrome caused by Hodgkin’s lymphoma in an adolescent girl: BMJ Case Rep, 2012; 2012; bcr0120125487
13.. Ramyar A, Shafiei M, Moazzami K, Rezaei N, Severe valvular toxicity and pericarditis early after radiation therapy in a patient treated for Hodgkin’s lymphoma: Turk J Pediatr, 2010; 52(4); 423-25
14.. Kentos A, Rocmans P, Remmelink M, Long-term remission with surgery for recurrent localized Hodgkin lymphoma: J Thorac Cardiovasc Surg, 2005; 129(5); 1172
15.. Saraya T, Shimura C, Mikura S, Huge mediastinal mass with SVC syndrome accompanying numerous chest wall collateral vessels: Intern Med, 2008; 47(19); 1719-22
16.. Yellin A, Superior vena cava syndrome associated with lymphoma: Arch Pediatr Adolesc Med, 1992; 146(9); 1060
17.. Kantarci M, Fil F, Bayraktutan U, Superior vena cava syndrome in a child and venous collateral pathways: MDCT imaging: J Thorac Oncol, 2008; 3(8); 915-16
18.. Kim YK, Sung YM, Hwang KH, Pseudopathologic vertebral body enhancement in the presence of superior vena cava obstruction on computed tomography: Spine J, 2015; 15(6); 1295-301
19.. Geller W, The mandate for chemotherapeutic decompression in superior vena caval obstruction: Radiology, 1963; 81(3); 385-87
20.. Bach AG, Schramm D, Surov A, A rare malposition of the central venous catheter: Heart, 2011; 97(23); 1992
21.. Pattaratuma V, Chandee T, Saiphoklang N, Anaesthetic management for caesarean delivery patient with obstructed superior vena cava by mediastinal mass: J Med Assoc Thai, 2016; 99(Suppl 8); S222-26
22.. Srinathan S, McCafferty I, Wilson I, Radiological management of superior vena caval stent migration and infection: Cardiovasc Intervent Radiol, 2005; 28(1); 127-30
23.. Eren S, Karaman A, Okur A, The superior vena cava syndrome caused by malignant disease: Eur J Radiol, 2006; 59(1); 93-103
24.. Adelstein DJ, Hines JD, Carter SG, Thromboembolic events in patients with malignant superior vena cava syndrome and the role of anticoagulation: Cancer, 1988; 62(10); 2258-62
25.. Porte H, Metois D, Finzi L, Superior vena cava syndrome of malignant origin. Which surgical procedure for which diagnosis?: Eur J Cardiothorac Surg, 2000; 17(4); 384-88
26.. Mehta SV, Koo DJ, Radiation-induced SVC syndrome: BMJ Case Rep, 2014; 2014; bcr2013203446
27.. Kostopoulou V, Tsiatas ML, Kelekis DA, Endovascular stenting for the management of port-a-cath associated superior vena cava syndrome: Emerg Radiol, 2009; 16(2); 143-46
28.. Davis RM, David E, Pugash RA, Radiofrequency guide wire recanalization of venous occlusions in patients with malignant superior vena cava syndrome: Cardiovasc Intervent Radiol, 2012; 35(3); 676-79
29.. Fichelle JM, Baissas V, Salvi S, [Thromboses ou sténoses de la veine cave supérieure sur chambres implantables. Six cas traités par voie endovasculaire ou chirurgie directe dans un contexte de cancer.]: J Med Vasc, 2018; 43(1); 20-28 [in French]
30.. Hasenclever D, Diehl V, A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease: N Engl J Med, 1998; 339(21); 1506-14
31.. Gisselbrecht C, Mounier N, André M, How to define intermediate stage in Hodgkin’s lymphoma?: Eur J Haematol Suppl, 2005(66); 111-14
32.. Yu JB, Wilson LD, Detterbeck FC, Superior vena cava syndrome – a proposed classification system and algorithm for management: J Thorac Oncol, 2008; 3(8); 811-14
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943420
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942824
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943118
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250