22 April 2021: Articles 
Breakthrough Bleeding Episodes at Minimum and Improvement in Quality of Life in a Child with Severe Hemophilia A with Inhibitors Treated with Emicizumab: A Case Report from Chile
Rare disease
Viviana Abarca-Villaseca1ABCDEF*, Verónica Soto-Arellano2ABCDEFDOI: 10.12659/AJCR.929598
Am J Case Rep 2021; 22:e929598
Abstract
BACKGROUND: People with hemophilia A have shown osteomuscular complications that have a significant impact on their quality of life (QoL) and on health care costs. Patients with hemophilia A with inhibitors living in developing countries such as Chile face a high disease and treatment burden. Emicizumab, a humanized bispecific monoclonal antibody, is associated with improvements in QoL and reduction in the financial impact of the disease related to treatment. This case report describes the impact of emicizumab on a patient with severe hemophilia A with inhibitors in terms of breakthrough bleeding control, improvements in QoL, and reduced financial impact after a year of treatment, in a country where this medication is not routinely available.
CASE REPORT: A 10-year-old child with severe hemophilia A with inhibitors had several restrictions in his daily life due to multiple incidences of breakthrough bleeding. He was on episodic treatment with bypassing agents, and in the year prior to treatment with emicizumab he had 18 bleeding episodes. After 1 year on prophylaxis treatment with emicizumab, the patient had only 1 bleeding episode (94.4% of reduction), improved pain control (5-point reduction on the visual analogue scale), a decrease in the Hemophilia Joint Health Score from 39 to 19, the QoL perception increased by 86% on the standardized Haemo-QoL-kids, and a 70% reduction in treatment costs versus the costs of episodic treatment with bypassing agents.
CONCLUSIONS: After 1 year of treatment with emicizumab, this patient had substantial improvements in the evaluated parameters. Further investigations with emicizumab are needed to assess its possible effects on public health policies.
Keywords: Blood Coagulation Factor Inhibitors, Hemophilia A, Quality of Life, Antibodies, Bispecific, Antibodies, Monoclonal, Humanized, Child, Chile, Factor VIII, Metrorrhagia
Background
Hemophilia is a genetic disease linked to the X chromosome that is expressed by a decrease in coagulation factor VIII or IX, corresponding to hemophilia A (HA) or B (HB), respectively. Hemophilia A affects one in 5,000–10,000 males every year [1]. Patients with this disease are prone to breakthrough bleeding with either spontaneous bleeding or traumatic bleeding episodes, particularly in the joints (elbow, knee, and ankle), that negatively affect quality of life (QoL) and provoke hemophilic arthropathy as a frequent complication [2].
Hemophilia is classified according to the percentage of deficiency in antihemophiliac factors, specifically, severe hemophilia (<1% of normal), moderate hemophilia (1–5% of normal), and mild hemophilia (5–40% of normal) [3,4]. Globally, data indicate that the cumulative incidence of inhibitor development in severe HA is in the range of 20–30% [5], while in different Latin American countries the prevalence of inhibitors is between 11% and 19% [6,7]. Affected patients have more annual bleeding events and osteomuscular complications than patients with other types of hemophilia, which affects QoL and has a significant impact on health care costs. This disease is challenging for patients, families, and the health care system [8].
The standard of care in Chile for every patient with the nonhemorrhagic phenotype is on-demand treatment only. For patients with the hemorrhagic phenotype, treatment corresponds to prophylaxis with bypassing agents (BPAs).
According to the HAVEN 2 study, once-weekly subcutaneous emicizumab prophylaxis resulted in a very low bleeding rate in children with HA and FVIII inhibitors; 77% of participants did not need treatment for bleeding episodes [9].
Case Report
INTERVENTION:
In the year before initiating treatment with emicizumab, the patient received episodic treatment with BPAs and had 18 bleeding episodes. Therefore, the emicizumab prophylactic treatment was started following the manufacturer’s recommendation for administration (loading dose: 3 mg/kg subcutaneously once weekly for 4 weeks; maintenance dose: 1.5 mg/kg subcutaneously once weekly).
PRIMARY VARIABLES:
A year before treatment with emicizumab began, the child’s weight was 24.5 kg and his height was 1.27 m; his body mass index was 15.1 kg/m2, placing him in the 34th percentile for children aged 8 years, indicating a healthy weight. After 1 year of using emicizumab, his weight was 34.4 kg and height 1.4 m, resulting in a body mass index of 17.5, putting the patient in the 66th percentile for children aged 10 years, indicating a healthy weight.
BLEEDING EPISODES:
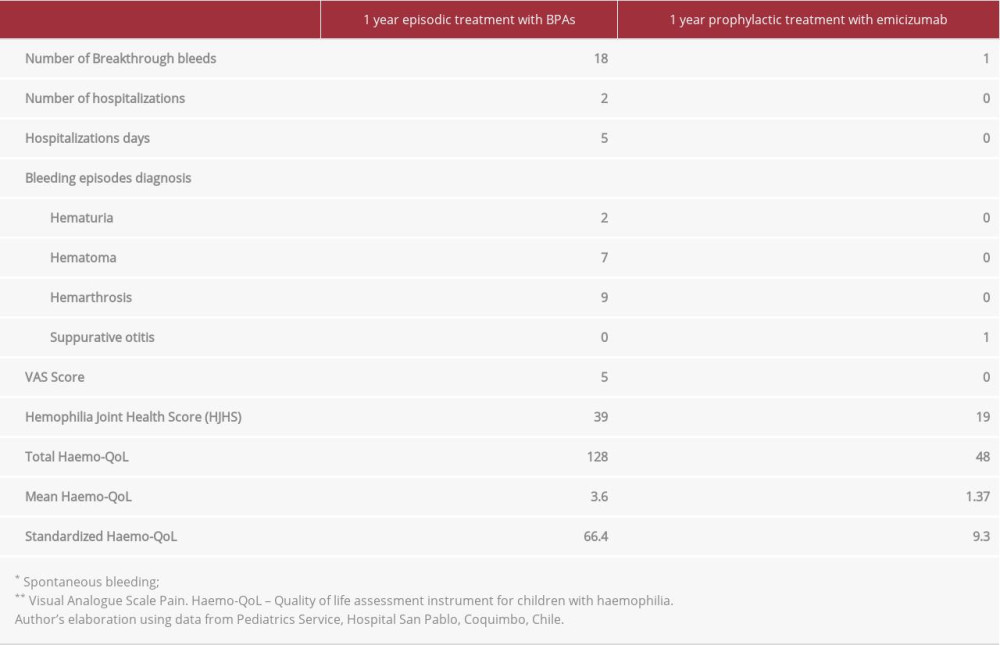
As mentioned previously, during the year before prophylaxis treatment with emicizumab started, the patient was treated with BPAs and had 18 bleeding episodes (all requiring attention in the Emergency Department). Two breakthrough bleeding episodes resulted in the patient’s hospitalization due to severe hematuria. Eight episodes were related to hemarthrosis, affecting the left ankle 6 times and the right and left elbow 1 time each. The other 8 episodes were related to hematoma, affecting the hand, thigh and right leg, right and left ankles, and big toe and left arm.
During the year of prophylactic treatment with emicizumab, the patient experienced only 1 bleeding episode, which did not require treatment with BPAs. It was a mild otorrhagia associated with suppurative otitis, which received the usual otitis treatment.
In a comparison of the 2 treatment regimens (ie, episodic treatment with BPAs vs prophylaxis with emicizumab) with regard to bleeding episodes, we found a reduction of 94.4% (18 vs 1). It is worth noting the significant 100% reduction in joint bleeds during the prophylaxis period with emicizumab, which was one of the greatest long-term cessation of joint bleeds that this patient has experienced.
ASSESSMENT OF JOINT HEALTH:
The HJHS tool was used to assess the patient’s joint health. During the episodic treatment year (7 months preceding the beginning of the emicizumab treatment) and after 1 year on prophylactic treatment with emicizumab, the scoring was 39 and 19, respectively.
ASSESSMENT OF PAIN:
Pain was assessed using the visual analogue scale (VAS) at 2 different times: at baseline (a full year of episodic treatment with BPAs) with a score of 5/10, and after 1 year on prophylactic treatment with emicizumab (VAS: 0/10). Both the patient and his caregivers considered him as being free of pain.
QUALITY OF LIFE: The short version of the Haemo-QoL instrument for children with hemophilia in the age range of 8 to 17 years of age, containing 35 items, scored on 9 scales was used to assess the QoL [10]. Scoring instructions for Haemo-QoL were analyzed according to the developer’s manual. Mean Haemo-QoL is the sum of coded items divided by 35, when at least 29 items have been answered; the scores obtained here were self-reported by the patient. The dimension values correspond to the standardized score, which falls in a range of 0 to 100. A high value indicates a poor QoL, while a lower value indicates a better QoL (Figure 1). The scores obtained were self-reported by the patient.
Primary variables are summarized in Table 1.
SECONDARY VARIABLES:
Direct and indirect costs were considered to estimate the economic burden.
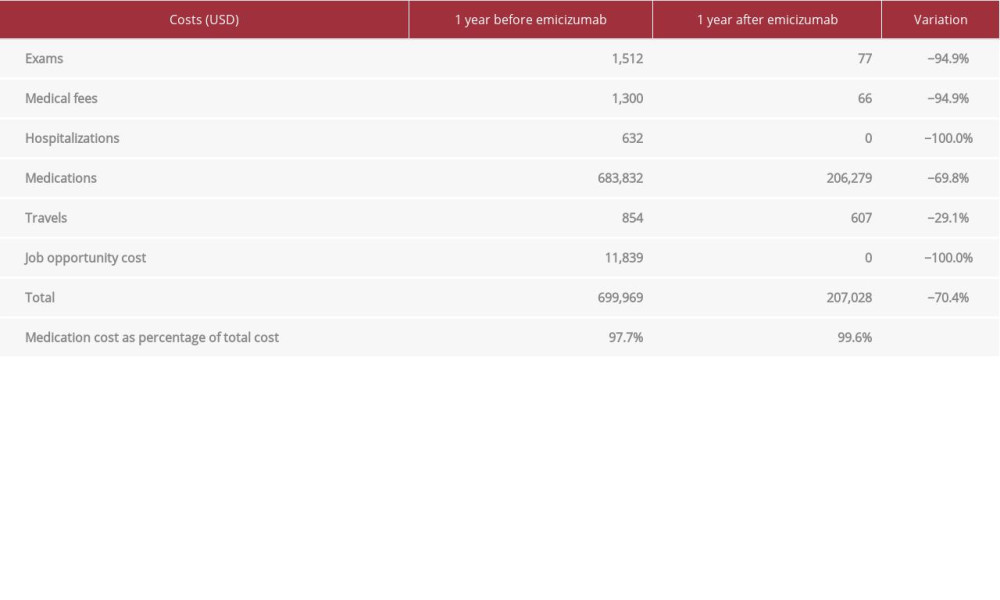
DIRECT COSTS: A total savings of US$492 941 (70%) was found in a comparison of the costs of prophylaxis with emicizumab versus the costs of treatment with episodic BPAs (Table 2). We analyzed the related costs in terms of treatments, laboratory tests, hospitalizations, and transfers to health institution. As reported in the literature, we also found that the vast majority of the total cost of the disease is associated with the coagulation factor concentrate used [11].
INDIRECT COSTS:
A total of US$15 388 was the increase in the household income after emicizumab was initiated, including savings due to the reduction in bleeding episodes. We considered the work downtime of the mother and the impact on elementary education. Before emicizumab, the mother was exclusively dedicated to childcare.
Regarding education issues, before using emicizumab, the child had hospital classroom support because he was not able to attend school. Once prophylactic treatment was initiated with emicizumab, he was able to attend to school in a usual way, and he was fully integrated in social activities with his peers.
Discussion
Initially, and due to the frequency of bleeding episodes, the patient described in this case report required continuous visits to the hospital to treat his symptoms. After his treatment with emicizumab, his bleeding episodes were reduced by 94.4%, which had a significant impact in terms of his improved QoL. All dimensions of the Haemo-QoL questionnaire showed a better perception of the child’s QoL after initiating treatment with emicizumab, highlighting the improvement in physical health, which has allowed the child to engage in normal activities of his age. Pain evolution assessed by applying the VAS went from a score of 5 (moderate) to a score of 0 (no pain). It should be noted that pain perception decreased, indicating imminent relief in his daily life. HJHS went from 39 to 19, between episodic and prophylactic periods, which is consistent with VAS descending and improvement of physical health and sport dimensions of QoL, improvements that will decrease long-term morbidity and hopefully decrease the need for costly orthopedic procedures when the patient is older. The standardized QoL score went from 66.4 (1 year before emicizumab) to 9.3 (1 year after emicizumab). This score evolution is mainly explained by the dimensions that showed the greatest improvement, as described in Figure 1. Regarding the
Department. The improvement in the
In terms of economic burden, the use of prophylaxis with emicizumab meant a 70% reduction in total costs versus the costs of the episodic treatment with BPAs. The elevated treatment costs explained practically the entire global cost of the disease, before and after the use of emicizumab in this child. Despite emicizumab having been shown to decrease the bleeding rate, access to this therapy in low-income countries in South America, specifically Chile, has not yet been systematically incorporated into treatment options due to the high cost of its use as a continuous prophylaxis therapy and because it has been on the market for only a short time. Treatment with emicizumab meant a significant reduction in disease total costs compared with previous treatments in this patient. Excluding treatment costs, the savings for the family was US$3548, which meant an 82% reduction compared with the period before using emicizumab. These data show that prophylaxis with emicizumab is a cost-saving measure compared with on-demand treatment with BPAs.
In this patient, the use of emicizumab had significant effects on pain evolution, improved QoL, and reduced the economic burden after 1 year of treatment.
The current standard practice in Chile for patients with HA with inhibitors is treatment with BPAs. The results obtained in this case report indicate that emicizumab is not only more cost-effective for this patient, but also for the health care system due to the immediate and likely long-term reduction in direct costs.
Emicizumab is not routinely available in Chile, where its implementation is limited due to concerns about the prohibitive cost. However, health care policy makers should consider the cost-effectiveness of this treatment. Initiating prophylaxis therapy with emicizumab for all patients with HA with inhibitors and for those in whom immunotolerance has failed or for those who do not have the opportunities to have it could generate considerable cost savings for the health care system.
The new World Federation of Hemophilia treatment guidelines indicate that patients with refractory inhibitors should be on prophylaxis, and emicizumab is the preferred treatment [5].
Conclusions
The data obtained from this study show that, after 1 year of treatment with emicizumab, bleeding events decreased to only 1 per year, the HJHS was reduced from 39 to 19, the intensity of pain was reduced by 5 points, QoL perception increased by 86%, and the total cost of the disease decreased by 70%. Further studies are required to confirm the findings presented in this report indicating that emicizumab prophylaxis in this patient population would be beneficial in countries that currently limit access due to financial concerns.
References:
1.. : Haemophilia Clinical Guide. MINSAL Clinical Guides Series, 2013, Santiago, Minsal [cited 2021 Mar 1]. https://www.minsal.cl/sites/default/files/Guia_Hemofilia.pdf
2.. Cassis FR, Buzzi A, Forsyth A, Haemophilia Experiences, Results and Opportunities (HERO) Study: Influence of haemophilia on interpersonal relationships as reported by adults with haemophilia and parents of children with haemophilia: Haemophilia, 2014; 20(04); e287-95
3.. Srivastava A, Brewer AK, Mauser-Bunschoten EP, Guidelines for the management of hemophilia: Haemophilia, 2013; 19(1); e1-47
4.. Blanchette VS, Key NS, Ljung LR, Definitions in hemophilia: Communication from the SSC of the ISTH: J Thromb Haemost, 2014; 12(11); 1935-39
5.. Srivastava A, Santagostino E, Dougall A, WFH guidelines for the management of hemophilia, 3rd edition: Haemophilia, 2020; 26(Suppl. 6); 1-158
6.. Rieger A, Roisenberg I, Prevalence of factor VIII inhibitors in patients with hemophilia A in Brazil: Thromb Haemost, 1999; 81; 475-76
7.. Wight J, Paisley S, The epidemiology of inhibitors in hemophilia A: A systematic review: Haemophilia, 2003; 9; 418-35
8.. Chen SL, Economic costs of hemophilia and the impact of prophylactic treatment on patient management: Am J Manag Care, 2016; 22; S126-33
9.. Young G, Liesner R, Chang T, A multicenter, open-label, phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors: Blood, 2019; 134(24); 2127-38
10.. von Mackensen S, Bullinger M, Development and testing of an instrument to assess the Quality of Life of Children with Haemophilia in Europe (Haemo-QoL): Haemophilia, 2004; 10(Suppl. 1); 17-25
11.. Duncan N, Roberson C, Lail A, A haemophilia disease management programme targeting costs and utilization of specialty pharmaceuticals: Haemophilia, 2014; 20; 519-26
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250