13 April 2021: Articles 
Spontaneous Bacterial Peritonitis in an Adult Patient with Minimal Change Disease
Rare disease
In Hee Lee1ABCDEF*, Hong Ik Kim1BCF, Min-Kyung Kim2BDE, Dong Jik Ahn3CDFDOI: 10.12659/AJCR.930677
Am J Case Rep 2021; 22:e930677
Abstract
BACKGROUND: Pediatric patients with nephrotic syndrome have a high risk of developing spontaneous bacterial peritonitis (SBP). However, SBP in adults with nephrotic syndrome is very rare. We report a case of SBP induced by Escherichia coli in a 60-year-old male patient on immunosuppressive therapy for the treatment of minimal change disease (MCD).
CASE REPORT: The patient was hospitalized with abdominal pain and generalized edema that had lasted for 2 weeks. The patient first started treatment with high-dose oral prednisolone after being diagnosed with MCD 6 months ago. Complete remission of nephrotic syndrome was not achieved even after 5 months of treatment. Thus, the treatment was changed to combination therapy with cyclosporine and low-dose prednisolone. At the time of admission, leukocytosis, hypoalbuminemia, decreased serum immunoglobulin G (IgG), azotemia, and nephrotic-range proteinuria were observed. Ascitic fluid analysis showed a leukocyte count of 4960/μL (neutrophils 90%). On the suspicion of SBP associated with MCD, intravenous administration of empirical cefotaxime and supportive therapy were initiated; however, symptoms of peritonitis persisted. Extended-spectrum beta-lactamase-negative E. coli was found in ascites cultures. Laparoscopy-assisted peritoneal biopsy revealed no evidence of fungal infection; however, chronic inflammation without granuloma formation was noted. Afterward, cefotaxime was changed to piperacillin-tazobactam. After 4 weeks of antibacterial therapy, the peritonitis was cured and renal function was improved.
CONCLUSIONS: Adult patients with steroid-resistant MCD accompanied by refractory ascites, severe hypoalbuminemia, and marked reduction in serum IgG are at a high risk of subsequent SBP and require careful monitoring.
Keywords: Nephrosis, Lipoid, nephrotic syndrome, peritonitis, Ascites, Ascitic Fluid, Bacterial Infections, Child, Escherichia coli, Liver Cirrhosis
Background
In patients with nephrotic syndrome, urinary excretion of proteins such as immunoglobulins and complement factors is increased, and the risk of infection increases due to complications such as lymphocyte dysfunction, administration of immunosuppressive agents, ascites, and edema [1]. The most common infectious complications include pneumonia, sepsis, cellulitis, and spontaneous bacterial peritonitis (SBP) [2]. SBP is a disease in which peritoneal bacterial inflammation occurs without apparent clinical evidence of infections in the abdominal cavity such as perforation of intraperitoneal organs, abscess, acute pancreatitis, or cholecystitis [3]. SBP is the most common infection in patients with advanced liver cirrhosis [3]. In addition, SBP is mainly observed in pediatric patients with nephrotic syndrome, and ascites predisposes to SBP development. Once developed, SBP greatly increases the rates of morbidity and mortality [2]. SBP is relatively rare in adult patients with nephrotic syndrome compared to pediatric patients with nephrotic syndrome. Thus, there are only sporadic reports on adults to date [4–9].
We report a case of extended-spectrum beta-lactamase (ESBL)-negative
Case Report
A 60-year-old man presented to the division of nephrology with chief complaints of abdominal pain and generalized edema, which had started 2 weeks earlier. The patient underwent a renal biopsy for nephrotic syndrome 6 months prior to the visit and was diagnosed with MCD (Figure 1A, 1B). Serum biochemistry at that time revealed a blood urea nitrogen (BUN) of 23.1 mg/dL, creatinine (Cr) 0.8 mg/dL, albumin 1.9 g/dL, and total cholesterol 371 mg/dL. The spot urine protein-to-Cr ratio was 8.864 g/g at that time. In the serum immunological tests, immunoglobulin G (IgG) level was decreased to 487 mg/dL (reference range, 700–1600 mg/dL); however, IgA and IgM levels were within the normal range. The concentrations of complement (C) 3 and C4 were within the normal range as well. Anti-streptolysin O, Venereal Disease Research Laboratory (VDRL) test, HBs Ag, and anti-HCV, anti-HIV, anti-nuclear, and anti-double stranded DNA antibodies were all negative. After MCD diagnosis, the patient started high-dose steroid therapy with oral prednisolone (60 mg/day, 1 mg/kg per day). However, complete remission was not achieved, and as generalized edema persisted even after 5 months of oral corticosteroids, steroid-resistant MCD was presumed. Therefore, 1 month prior to presentation, the treatment was changed to cyclosporine (300 mg/day, 5 mg/kg per day) in combination with low-dose oral prednisolone (10 mg/day).
At the time of admission, blood pressure, pulse rate, respiratory rate, and body temperature were 140/90 mmHg, 64/min, 21/min, and 37.8ºC, respectively. The patient was conscious but had an acutely ill appearance. He had no history of hypertension, diabetes mellitus, or liver or cardiac diseases. Abdominal examination showed marked distension of the abdomen with no hepatosplenomegaly. Diffuse tenderness, but no rebound tenderness, was noted. Moderate pitting edema was present in both lower extremities. A peripheral blood test showed a white blood cell (WBC) count of 13 700/μL (neutrophils 94.5%), hemoglobin 13.1 g/dL, platelet count 245 000/μL; and erythrocyte sedimentation rate 96 mm/h. Serum biochemical examination revealed BUN 104 mg/dL, Cr, 3.2 mg/dL (estimated glomerular filtration rate 20 mL/min/1.73 m2), total protein 4.4 g/dL, albumin 1.7 g/dL, total cholesterol 753 mg/dL, and C-reactive protein (CRP) 68.1 mg/L (reference range <5 mg/L). Urinalysis with microscopic examination showed albumin 3+, a WBC count of 5–9/high-power field (HPF), and a red blood cell (RBC) count of 10–30/HPF (90% dysmorphic). The spot urine protein-to-Cr ratio was 6.998 g/g. Serum IgG concentration was significantly reduced to 420 mg/dL. Abdominal computed tomography revealed no abnormality except massive ascites and intestinal wall edema. Paracentesis showed cloudy ascites, and ascitic fluid analysis showed WBC of 4960/μL (neutrophils 90%), RBC 720/μL, and lactate dehydrogenase 341 IU/L. As SBP was suspected, ascites was drained, and an empirical broad-spectrum antibiotic (cefotaxime) was administered intravenously (2.0 g, 2×/day).
On the fifth hospital day, ESBL-negative
Discussion
SBP is one of the major complications in pediatric patients under the age of 10 years with nephrotic syndrome [2]. Indicators of SBP include an absence of infectious lesions in the perito-neal cavity requiring surgical treatment and neutrophils >250/ μL in the ascites [3]. The incidence rate of SBP is 2–6% in pediatric patients with nephrotic syndrome, and the mortality rate is reported to be 1.5% [2]. SBP usually occurs within 2 years after the diagnosis of nephrotic syndrome and rarely appears as the first symptom of nephrotic syndrome. However, SBP has been discovered as a complication at the time of recurrence of nephrotic syndrome [10,11].
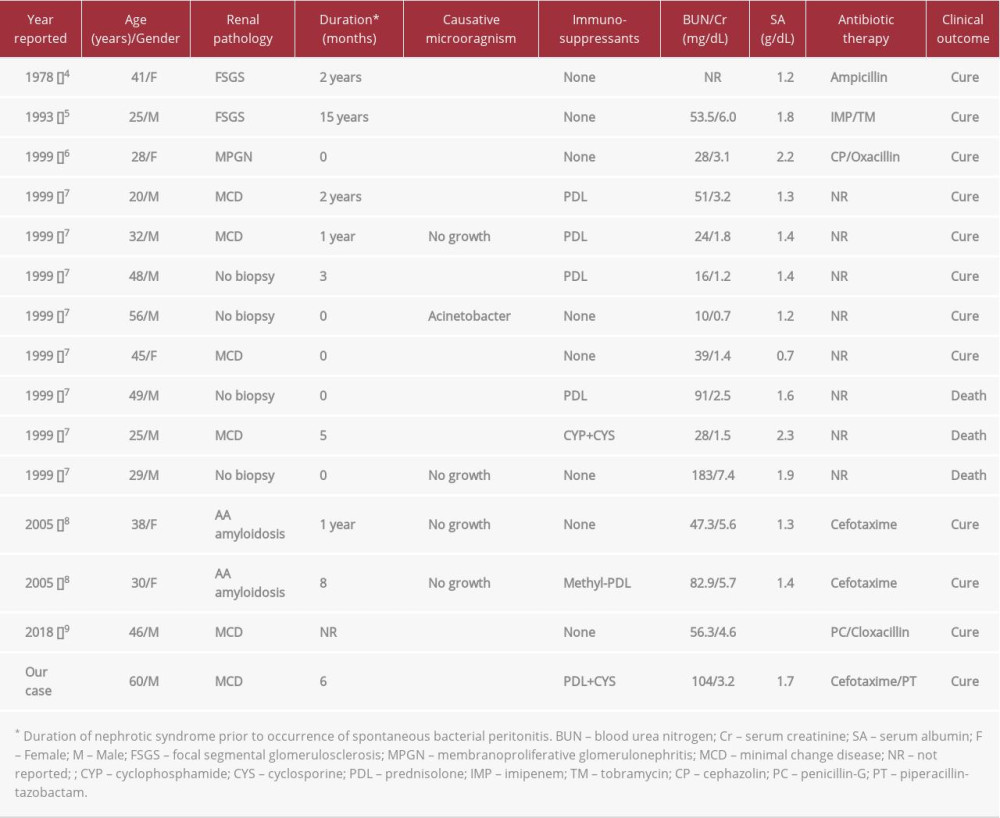
SBP in adult patients (>18 years old) with nephrotic syndrome is very rare compared to pediatric patients. Since the report of the first case in 1978 [4], 14 cases have been reported in the English literature (Table 1). Our retrospective analysis of these cases shows a male-to-female ratio of 1.8: 1 and a mean age of 36.6 years (range 20–56 years) at the time of diagnosis. Although there were 4 cases without pathological confirmation, MCD was the most common cause of nephrotic syndrome (5/14; 35.7%), followed by focal segmental glomerulosclerosis (2/14), amyloidosis (2/14), and membranoproliferative glomerulonephritis (1/14). SBP mostly occurred within 2 years of diagnosis of nephrotic syndrome, which was similar to the occurrence in pediatric patients. In particular, 5 cases (5/14; 35.7%) of peritonitis occurred simultaneously or within the first month of diagnosis of nephrotic syndrome [6,7].
SBP occurrence in patients with nephrotic syndrome is associated with a number of risk factors [6]. In patients with nephrotic syndrome, serum opsonic activity and phagocytosis of encapsulated microorganisms decrease due to the decreased levels of serum IgG and complement factors B and D [13,14]. In addition, T-lymphocyte dysfunction increases the susceptibility to bacterial infections [15]. Hypoalbuminemia leads to a secondary increase in ascites production and a diluent decrease in the concentration of complements and immunoglobulins in the ascites, thereby reducing bactericidal activity in the perito-neal cavity. Moreover, bowel wall edema and venous congestion of the gastrointestinal tract may facilitate intraperitoneal permeation of intestinal bacteria [16,17]. The administration of immunosuppressive agents for the treatment of nephrotic syndrome is also suggested as a major factor in the development of peritonitis. Among previous cases of adult nephrotic syndrome, 6 patients (6/14; 42.8%) were on immunosuppressive therapy at the time of SBP onset, and 5 of these 6 patients were being treated with corticosteroids (Table 1). However, a study reported that only 50% of pediatric patients with nephrotic syndrome and SBP were on steroid therapy when diagnosed with peritonitis [18]. Therefore, the clinical association between the administration of immunosuppressive agents and SBP is not yet established.
The most common bacterial species that cause SBP in pediatric nephrotic patients include
It is also unclear why SBP is more common in pediatric patients with nephrotic syndrome than in adults. Possible clinical explanations include that MCD, in which cellular and humoral immune systems are markedly impaired, is more common in children, and ascites formation along with severe hypoalbuminemia is more pronounced in pediatric nephrotic syndrome [6]. In a study comparing the clinical characteristics of 52 adults and 21 children with nephrotic syndrome, ascites was observed in 23% and 52%, respectively, and the mean serum albumin concentration was significantly lower in pediatric patients with ascites than in adult patients with ascites (1.7 g/dL vs 2.1 g/dL) [17]. Furthermore, in adults with nephrotic syndrome, congestive heart failure and chronic liver disease, which lead to an increase in hepatic sinusoidal pressure in addition to hypoalbuminemia, may increase ascites production [17]. A marked decrease in serum albumin (≤1.5 g/dL) is known as a significant risk factor predicting the development of SBP in patients with nephrotic syndrome. However, the classic markers indicative of active renal diseases, such as hypertension and microscopic hematuria, were not associated with the occurrence of peritonitis [10]. This may suggest that the incidence of SBP associated with nephrotic syndrome can be reduced if clinical remission is achieved with optimal treatment. In our analysis of published cases of adult nephrotic syndrome with SBP, renal dysfunction (serum Cr >1.4 mg/dL) and severe hypoalbuminemia (serum albumin <2.0 g/dL) were present in most patients (Table 1).
Azotemia accompanied by SBP development is presumed to be related to prerenal acute kidney injury caused by hypoalbuminemia and/or endogenous renal insufficiency secondary to intraabdominal inflammation. Renal dysfunction was actually improved to the normal level in most patients after cure of SBP. However, 1 case of irreversible renal failure even after amelioration of SBP with antibiotic therapy [5] and 3 cases of in-hospital mortality from septic shock during peritonitis treatments have been reported [7]. Therefore, prompt and appropriate treatment and careful follow-up are needed on suspicion of SBP. Significantly reduced serum IgG (<600 mg/dL) and renal insufficiency (serum Cr >2.0 mg/dL) were reported as independent risk factors for the development of bacterial infection in adult patients with nephrotic syndrome [20]. In our case, congestive heart failure and chronic liver disease were not identified in the patient, and there were no localized infections or other clinical evidence suggestive of secondary peritonitis. Therefore, it can be inferred that the decrease in serum albumin (1.7 g/dL), low serum IgG (420 mg/dL), and renal dysfunction were significantly associated with the development and persistence of SBP.
Conclusions
This case report describes an adult MCD patient who developed
Figures
References:
1.. Wang CS, Greenbaum LA, Nephrotic syndrome: Pediatr Clin North Am, 2019; 66(1); 73-85
2.. Eddy AA, Symons JM, Nephrotic syndrome in childhood: Lancet, 2003; 362(9384); 629-39
3.. Dever JB, Sheikh MY, Review article: Spontaneous bacterial peritonitis – bacteriology, diagnosis, treatment, risk factors and prevention: Aliment Pharmacol Ther, 2015; 41(11); 1116-31
4.. Rusthoven J, Kabins SA, Hemophilus influenzae f cellulitis with bacteremia, peritonitis, and pleuritis in an adult with nephrotic syndrome: South Med J, 1978; 71(11); 433-35
5.. Kato A, Ohtake T, Furuya R, Spontaneous bacterial peritonitis in an adult patient with nephrotic syndrome: Intern Med, 1993; 32(9); 719-21
6.. Chuang TF, Kao SC, Tsai CJ, Spontaneous bacterial peritonitis as the resenting feature in an adult with nephrotic syndrome: Nephrol Dial Transplant, 1999; 14(1); 181-82
7.. Chen MC, Lam KK, Hsu KT, Spontaneous bacterial peritonitis in adult patients with primary nephrotic syndrome: Changgeng Yi Xue Za Zhi, 1999; 22(2); 227-33
8.. Danis R, Ozmen S, Yilmaz S, Adult nephrotic syndrome with spontaneous bacterial peritonitis: Hong Kong J Nephrol, 2005; 7; 90-92
9.. Makedonov I, Clark EG, Kanchi P, Adult nephrotic syndrome complicated by spontaneous pneumococcal peritonitis: A case report: Clin Nephrol, 2018; 90(1); 76-78
10.. Hingorani SR, Weiss NS, Watkins SL, Predictors of peritonitis in children with nephrotic syndrome: Pediatr Nephrol, 2002; 17(8); 678-82
11.. Uncu N, Bülbül M, Yildiz N, Primary peritonitis in children with nephrotic syndrome: Results of a 5-year multicenter study: Eur J Pediatr, 2010; 169(1); 73-76
12.. Vivarelli M, Massella L, Ruggiero B, Minimal change disease: Clin J Am Soc Nephrol, 2017; 12(2); 332-45
13.. Yokoyama H, Kida H, Abe T, Impaired immunoglobulin G production in minimal change nephrotic syndrome in adults: Clin Exp Immunol, 1987; 70(1); 110-15
14.. Matsell D, Wyatt RJ, The role of I and B in peritonitis associated with the nephrotic syndrome: Pediatr Res, 1993; 34(1); 84-88
15.. Goonewaedene ST, Tang C, Tan LT, Safety and efficacy of pneumococcal vaccination in pediatric nephrotic syndrome: Fron Pediatr, 2019; 13(7); 339
16.. Akalin HE, Fisher KA, Lalelli Y, Bactericidal activity of ascitic fluid in patients with nephrotic syndrome: Eur J Clin Invest, 1985; 15(3); 138-40
17.. Ackerman Z, Ascites in nephrotic syndrome: Incidence, patient’s characteristics, and complications: J Clin Gastroenterol, 1996; 22(1); 31-34
18.. Feinstein EI, Chesney RW, Zelikovic I, Peritonitis in childhood renal disease: Am J Nephrol, 1988; 8(2); 147-65
19.. Soni H, Kumar-M P, Sharma V, Antibiotics for prophylaxis of spontaneous bacterial peritonitis: Systemic review & Bayesian network meta-analysis: Hepatol Int, 2020; 14(3); 399-413
20.. Ogi M, Yokoyama H, Tomosugi N, Risk factors for infection and immunoglobulin replacement therapy in adult nephrotic syndrome: Am J Kidney Dis, 1994; 24(3); 427-36
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250