28 April 2021: Articles 
Glycemic Profile of Intravenous Dexamethasone-Induced Hyperglycemia Using Continuous Glucose Monitoring
Unusual clinical course, Unusual or unexpected effect of treatment, Educational Purpose (only if useful for a systematic review or synthesis)
Fan Zhang12ABCDEF*, Jocelyne G. Karam13ABCDEFDOI: 10.12659/AJCR.930733
Am J Case Rep 2021; 22:e930733
Abstract
BACKGROUND: Intravenous (IV) dexamethasone is widely used in critical illness, chemotherapy, or severe COVID-19. Although glucocorticoid-induced hyperglycemia (GCIH) is well-known, there is no report describing the glycemic profile following a single dose of IV dexamethasone as captured on continuous glucose monitoring (CGM) in a patient with diabetes treated with insulin.
CASE REPORT: A 70-year-old woman with diabetes and pancreatic adenocarcinoma was treated with chemotherapy containing dexamethasone every other week. CGM data of 23 cycles revealed a reproducible triphasic glycemic pattern consisting of a constant hyperglycemia period, followed by a transient improvement, and ending with another hyperglycemic plateau. Given this recurrent pattern, basal insulin and correction insulin were adjusted with subsequent GCIH attenuation.
CONCLUSIONS: This is the first report of CGM glycemic profile following recurring doses of IV dexamethasone in a patient with diabetes treated with basal-bolus insulin. The understanding of triphasic glycemic pattern allows optimal glycemic management.
Keywords: Blood Glucose, Dexamethasone, Diabetes Mellitus, Glucocorticoids, Hyperglycemia, Insulin, continuous glucose monitoring, Administration, Intravenous, Antineoplastic Agents, Hormonal, Blood Glucose Self-Monitoring, COVID-19, Diabetes Mellitus, Type 2, Hypoglycemic Agents, Pancreatic Neoplasms, SARS-CoV-2
Background
Glucocorticoids are widely used for their anti-inflammatory and immunosuppressive properties, in addition to their adjuvant effects in chemotherapy [1]. Dexamethasone, a potent long-acting glucocorticoid, is also commonly used for critically ill patients and was recently found to lower the mortality rate of patients with severe COVID-19 pneumonia [2]. One of the major adverse effects of glucocorticoids is hyperglycemia, which is challenging to manage in patients with diabetes and comorbidities. Steroid-induced hyperglycemia is common in patients treated for cancer, and glycemic control is crucial since hyper-glycemia worsens morbidity and mortality [3–5]. Numerous efforts have been made to identify the empirically and theoretically optimal regimen for GCIH based on the typical glycemic profiles [6–10]. However, the actual glucose trending pattern following 1 dose of IV dexamethasone is unclear [6].
The increased availability of CGM allowed a detailed description of glycemic fluctuations. Several studies investigated hyperglycemia following more than 3 days of daily dose dexamethasone treatment [11–13], but no report documented the CGM pattern following a single dose of IV dexamethasone in a patient with diabetes mellitus (DM) on insulin. We observed the daily glycemic profiles of a patient with DM and CGM for 23 cycles of biweekly chemotherapy containing 1 dose of IV dexamethasone for more than 1 year [14]. A reproducible triphasic glycemic pattern was discovered and the insulin regimen was tailored accordingly. This finding provides information to predict glucose trends and enable optimal glycemic control for patients requiring IV dexamethasone treatment.
Case Report
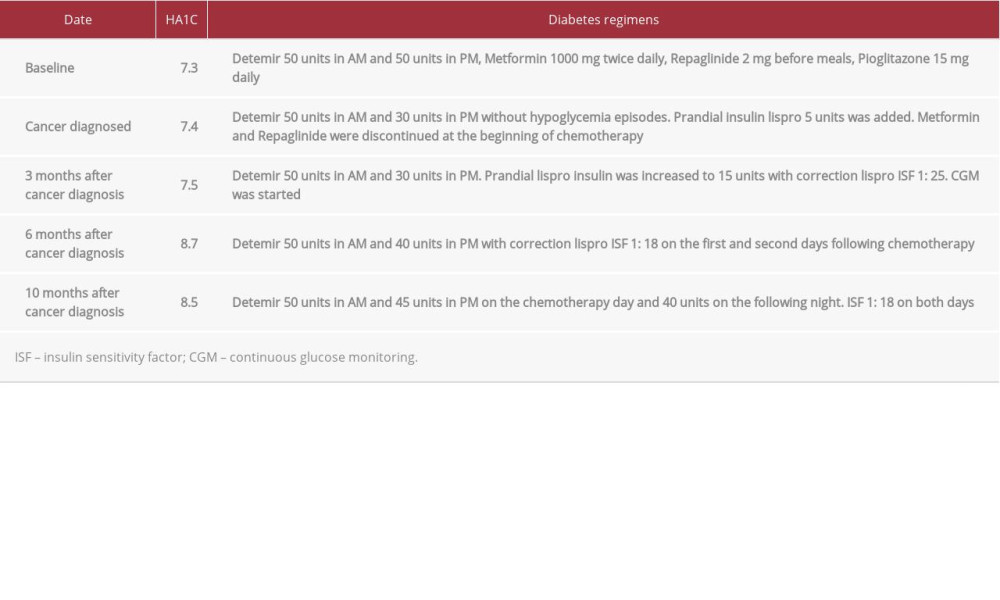
The patent was a 70-year-old woman with a 19-year history of type 2 DM complicated by retinopathy, peripheral neuropathy, coronary artery disease, and metastatic pancreatic adeno-carcinoma. Her diabetes was previously fairly controlled with HA1C 7.4% on metformin 1000 mg twice daily, repaglinide 2 mg before meals, pioglitazone 15 mg daily, and long-acting insulin detemir 50 units every 12 h. Upon diagnosis of pancreatic adenocarcinoma metastasis to the lungs and omentum, she experienced significant weight loss, poor appetite and hypoglycemia episodes. Positron emission tomography–computed tomography (PET-CT) revealed a 2-cm focal uptake in the pancreatic body, some ill-defined density on the omentum, subpleural nodules, and multiple pulmonary nodules. The detemir dose was decreased to 50 units in the AM and 30 units in the PM, with no more hypoglycemia episodes. Prandial insulin lispro 5 units was added while metformin and repaglinide were discontinued at the beginning of chemotherapy, which consisted of gemcitabine 2200 mg IV, paclitaxel 220 mg IV, and dexamethasone 8 mg IV on every other Wednesday. Freestyle Libre (Abbott Laboratories) CGM was started and prandial lispro insulin was increased to 15 units with an additional 2 units for every 50 mg/dL glucose above the target glucose as a correction using an insulin sensitivity factor (ISF) of 1: 25. Based on the CGM reports, a higher insulin regimen was prescribed on her chemotherapy day, with detemir 50 units in the morning and 40 units at night, and a correction lispro scale of 3 units for every 50 mg/dL glucose above 200 mg/dl on the first and second day following chemotherapy. Her chemotherapy regimen was changed to fluorouracil 2700 mg and a higher-dose IV dexamethasone 12 mg. Subsequent CGM showed a fairly controlled glucose level on days off steroids, but significant hyperglycemia following the chemotherapy, despite usage of a higher-insulin regimen. HA1C level increased to 8.7% with normal liver function, renal function (creatinine 0.8 mg/dL), hemoglobin level, and stable body weight.
A closer look at CGM reports revealed a reproducible triphasic glycemic pattern following IV dexamethasone, consisting of a steady state of hyperglycemia reached within 3 h and lasting around 23–35 h until the afternoon of the next day (Figure 1). Unlike the known postprandial hyperglycemia exacerbation with steroids, CGM demonstrated steady hyperglycemia unrelated to meals. A subsequent transient blood glucose (BG) improvement for 14–21 h was followed by another hyperglycemic plateau of 7–13 h on day 3 after chemotherapy, with less amplitude than on the first 2 days but a higher glucose level compared to her usual days. BG gradually trended down back to normal after 3 days (Figure 2). The pattern and amplitude of hyperglycemia were strikingly similar after different biweekly doses of dexamethasone therapy over several months. Given this recurrent pattern, the bedtime detemir insulin was increased from 30 to 45 units on the chemotherapy day and 40 units on the following night. Her correction insulin ISF was increased from 1: 25 to 1: 18 on both days. CGM showed subsequent attenuation and shortening of GCIH. The timeline of insulin dose adjustment was correlated with the chemotherapy and HA1C level (Table 1).
Discussion
Glucocorticoids are used to treat a wide range of inflammatory disease, critical illnesses, and neoplastic conditions. Dexamethasone 6 mg IV once daily was also found to lower mortality in hospitalized COVID-19 patients [2]. One of the common adverse effects of glucocorticoid is hyperglycemia, which occurs in 20–50% of patients without a previous history of diabetes and 46–100% of diabetic patients due to more severe beta cell defect and greater impairment of postprandial insulin release [15–17]. Uncontrolled hyperglycemia is associated with infection, prolonged length of hospital stays, increased mortality, chemotoxicity, and poor clinical outcome in malignancies [11].
The knowledge of the glycemic pattern of GCIH is crucial to allow a tailored insulin regimen. CGM measures interstitial glucose concentration with a short time lag and provides glycemic profile, especially nocturnal and postprandial glycemia, which are usually unchecked by standard point-of-care glucose testing. No previous studies have described detailed CGM hyper-glycemia profiles following 1 dose dexamethasone IV treatment in patients with DM on insulin. We report for the first time a consistent triphasic glycemic pattern revealed by CGM following 23 cycles chemotherapy containing dexamethasone.
Risks for GCIH include abdominal adiposity, genetic predisposition to diabetes, age, high body mass index, higher glucocorticoid doses, treatment duration, and systemic inflammation marked by CRP level [16,18]. The mechanisms of steroid-induced hyperglycemia were found to be associated with increased peripheral insulin resistance and hepatic glucose production, suppressed pancreatic beta cell insulin production, enhanced glucagon effect, and stimulated protein catabolism generating amino acids as precursors for gluconeogenesis [15,19]. The impaired glucose uptake in peripheral tissues, such as a post-receptor effect of glucose transport and glycogen synthesis in muscle and adipose tissue, was deemed as the major underlying pathophysiology of glucocorticoid-induced postprandial hyperglycemia. The reduction of insulin secretion was observed at higher doses or with long-acting glucocorticoid due to increased intracellular calcium concentration [20].
Many studies have investigated GCIH management based on the steroid type, dosage, duration, and severity of hyperglycemia. GCIH usually occurs 3–4 h following administration of intermediate-acting glucocorticoid, with a half-life of 12-16 h, such as prednisone, prednisolone and methylprednisolone, and peaks at approximately 4-6 h, with typical postprandial glycemic excursions lasting 12–16 h [1,16]. If oral prednisone is administered in the morning to mimic the physiological diurnal rhythm, hyperglycemia, characterized as predominantly post-prandial, occurs in the afternoon, and gradually wears off at bedtime, with fasting glucose remaining normal or slightly elevated the next morning, similar to the postprandial hyper-glycemia in Cushing’s syndrome. Therefore, the glucose-lowering therapy should target the time after lunch and dinner. However, the long-acting glucocorticoid, dexamethasone, is more potent and lasts longer, with hyperglycemia starting in about 3 h, peaking around 9–10 h, and remitting 48 h after discontinuation [7].
Our patient’s CGM data showed a reproducible triphasic glycemic pattern following IV dexamethasone, consisting of a steady state of hyperglycemia reached within 3 h and lasting around 23–35 h, followed by a transient BG improvement for 14–21 h, and ending with another hyperglycemic plateau of 7–13 h on day 3 after chemotherapy, with no association to meal intake (Figure 1A). The prolonged hyperglycemia during the first 30 h may reflect the persistent insulin resistance throughout the day rather than only a postprandial defect [7]. The second hyperglycemia phase is most likely related to the impaired glucose uptake in peripheral tissues and insulin secretion related postprandial hyperglycemia shown by the similar glycemic pattern between day 3 and other days of the patient’s usual state (Figure 2). The transient improvement of hypoglycemia between the 2 hyperglycemia phases may be due to the inhibited endogenous glucocorticoids secretion along with the dampened dexamethasone effects, with a half-life of 20–36 h [1] and apparently intentional reduction of carbohydrates intake by the patient in response to initial hyper-glycemia. It indicates a higher risk of nocturnal hypoglycemia by long-acting basal insulin (Figure 1B). Further investigation of the underlying mechanism will provide insights for future management targets.
Management of hyperglycemia in diabetic patients with hematologic malignancies during dexamethasone therapy for 3 days (IV 8–12 mg/day or oral 40 mg/day) was studied by Gosmanov et al [11]. They found that a basal and bolus insulin (BBI) regimen is a more effective and safer approach compared with sliding-scale regular insulin (SSI). Interestingly, the glycemic trend and outcome were identical in both the IV and oral groups among the 40 patients, suggesting that high-dose dexamethasone has equal hyperglycemic effects, independent of dose and route of administration [11]. Unfortunately, the precise glucose profile was unknown since the data were obtained from daily average glucose levels. In our case, CGM revealed the GCIH pattern and confirmed that the dose of dexamethasone (8 mg or 12 mg) has no differential effect on the amplitude of hyperglycemia.
Kishimoto et al reported a case of CGM in a diabetic patient treated with 20 mg dexamethasone as a part of therapy for multiple myeloma [21]. Similar to our observation, CGM showed hyperglycemia mainly in the afternoon and lasting to the following day for approximately 30 h without wearing off overnight, even after being treated with regular insulin 4–10 units every 6 h. In contrast with our case, that patient’s diabetes had required therapy with only oral agents and not insulin.
This would explain why only mildly higher glucose levels were observed on the third day compared to the baseline, whereas the elevation was much more dramatic in our patient, who required insulin, based on more extended insulin insensitivity or metabolism defect. A basal-bolus insulin regimen was used in our case instead of regular insulin as per current guidelines. Furthermore, our observation of CGM in multiple cycles provided reproducible data sufficient to demonstrate the glycemic pattern. HA1C increased from 7.4% to 8.7% after starting chemotherapy due to GCIH by dexamethasone. After adjusting the insulin dose based on the CGM pattern, HA1C started slowly trending down to 8.5% (Table 1).
It is recommended to treat GCIH with basal-bolus insulin regimen with starting dosage of 0.3 to 0.5 U/kg daily [15]. Correctional insulin regimens based on different steroid types, doses, and body weight were proposed to be used in conjunction with basal-bolus insulin to match the different glycemic profiles associated with the glucocorticoid administered [7]. Isophane insulin (NPH) is recommended for prednisolone-induced hyperglycemia based on its pharmacokinetic pattern that matches the distinct circadian pattern of hyperglycemia predominantly in the afternoon and evening [6]. The distribution of preprandial insulin boluses was also adjusted to less for breakfast and more for lunch and afternoon snack [8]. Regular insulin is more suitable for use with a short-acting steroid, hydrocortisone, due to similar peak in 2 h, whereas for the prolonged steady hyperglycemia induced by the twice-daily prednisolone or dexamethasone, a long-acting basal insulin, glargine or detemir, has a better match based on the flatter insulin profile [1,7].
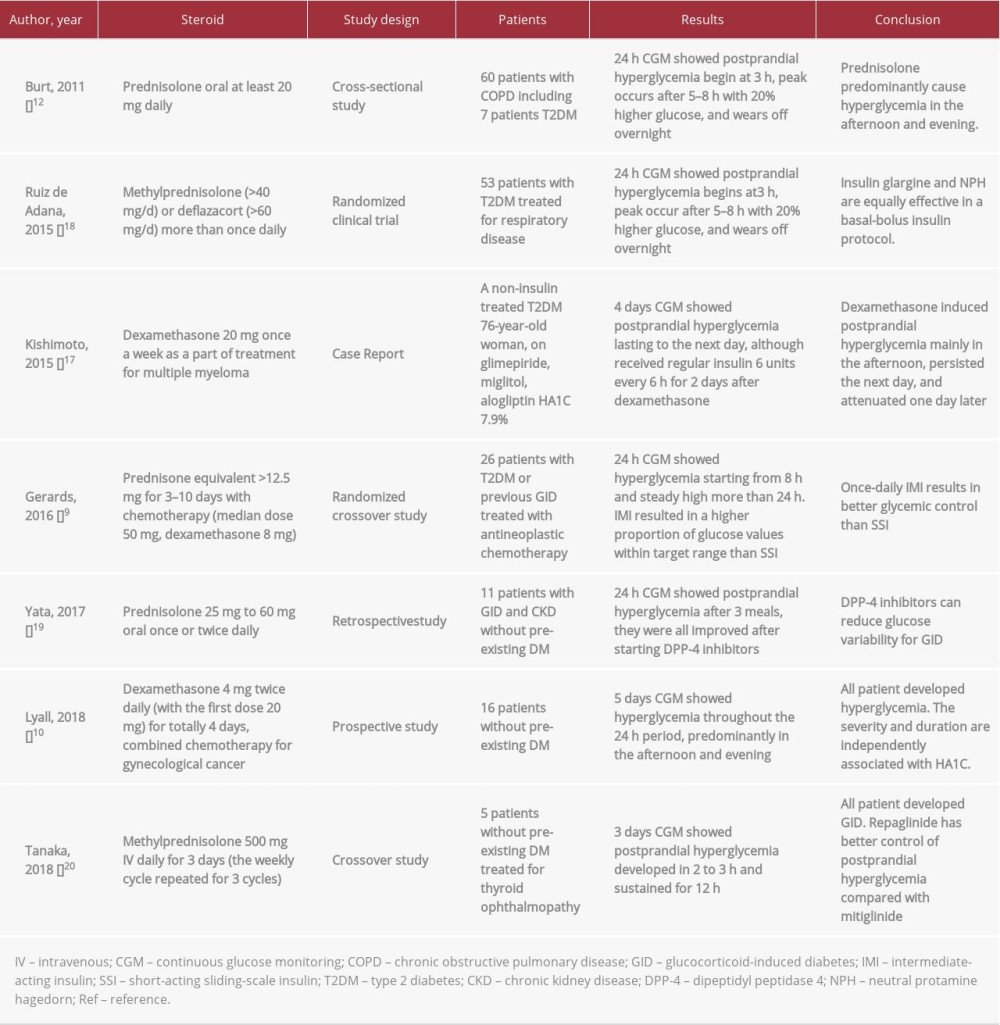
CGM was used in several studies to investigate the efficacy and safety of hypoglycemic regimen for different types of glucocorticoids [22–24]. Table 2 provides a summary of studies using CGM for GCIH. However, the glycemic pattern and insulin doses required in dexamethasone-treated patient have not been well described. Our report is applicable to a single-injection dexamethasone regimen. The glucose dynamics may be different in patients receiving multiple doses of dexamethasone. Although in our patient the pancreatic lesion was only 2 cm on PET-CT, there could be a potential impact of pancreatic cancer on the endogenous beta cell function and glucagon response to hypoglycemia. Therefore, patients with other malignancies might require different insulin dosing strategies to control blood glucose levels, but their patterns of dexamethasone-induced hyperglycemia might be very similar. Further studies can expand on the findings of our study. Ultimately, a good knowledge of the glycemic profile is crucial to successfully treat the patient with the appropriate insulin regimen.
Conclusions
Our case is the first report of a reproducible triphasic glycemic pattern following a single dose of IV dexamethasone documented by CGM in a patient with diabetes treated with insulin. The CGM data from 23 cycles of chemotherapy containing dexamethasone revealed a steady state of hyperglycemia reached within 3 h and lasting around 29 h, followed by a transient BG improvement, and ending with another hyperglycemic plateau before trending back to normal on the third day following dexamethasone administration. Understanding the precise glycemic profiles of GCIH in patients with diabetes enables tailoring an insulin regimen for optimal glycemic control.
Figures
References:
1.. Perez A, Jansen-Chaparro S, Saigi I, Glucocorticoid-induced hyperglycemia: J Diabetes, 2014; 6(1); 9-20
2.. Horby P, Lim WS, Dexamethasone in hospitalized patients with COVID-19: N Engl J Med, 2021; 384(8); 693-704
3.. Barua R, Templeton AJ, Seruga B, Hyperglycaemia and survival in solid tumours: A systematic review and meta-analysis: Clin Oncol (R Coll Radiol), 2018; 30(4); 215-24
4.. Umpierrez GE, Isaacs SD, Bazargan N, Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes: J Clin Endocrinol Metab, 2002; 87(3); 978-82
5.. Harris D, Barts A, Connors J, Glucocorticoid-induced hyperglycemia is prevalent and unpredictable for patients undergoing cancer therapy: An observational cohort study: Curr Oncol, 2013; 20(6); e532-38
6.. Radhakutty A, Burt MG, Management of endocrine disease: Critical review of the evidence underlying management of glucocorticoid-induced hyper-glycaemia: Eur J Endocrinol, 2018; 179(4); R207-18
7.. Lakhani OJ, Kumar S, Tripathi S, Comparison of two protocols in the management of glucocorticoid-induced hyperglycemia among hospitalized patients: Indian J Endocrinol Metab, 2017; 21(6); 836-44
8.. Agudo-Tabuenca A, Gimeno-Orna JA, Saenz-Abad D, Assessment of the efficacy and safety of a protocol to manage glucocorticoid-induced hyperglycemia in diabetic patients during hospital stay: Endocrinol Diabetes Nutr, 2019; 66(6); 353-60
9.. Tatalovic M, Lehmann R, Cheetham M, Management of hyperglycaemia in persons with non-insulin-dependent type 2 diabetes mellitus who are started on systemic glucocorticoid therapy: A systematic review: BMJ Open, 2019; 9(5); e028914
10.. Bonaventura A, Montecucco F, Steroid-induced hyperglycemia: An under-diagnosed problem or clinical inertia? A narrative review: Diabetes Res Clin Pract, 2018; 139; 203-20
11.. Gosmanov AR, Goorha S, Stelts S, Management of hyperglycemia in diabetic patients with hematologic malignancies during dexamethasone therapy: Endocr Pract, 2013; 19(2); 231-35
12.. Gerards MC, de Maar JS, Steenbruggen TG, Add-on treatment with intermediate-acting insulin versus sliding-scale insulin for patients with type 2 diabetes or insulin resistance during cyclic glucocorticoid-containing antineoplastic chemotherapy: A randomized crossover study: Diabetes Obes Metab, 2016; 18(10); 1041-44
13.. Lyall MJ, Thethy I, Steven L, Diurnal profile of interstitial glucose following dexamethasone prophylaxis for chemotherapy treatment of gynaecological cancer: Diabet Med, 2018; 35(11); 1508-14
14.. Zhang F, Goldberg Z, Karam JG, SAT-640 glycemic profile of intravenous glucocorticoid induced hyperglycemia using continuous glucose monitoring: J Endocr Soc, 2020; 4(Suppl. 1); SAT-640
15.. Umpierrez GE, Hellman R, Korytkowski MT, Management of hyperglycemia in hospitalized patients in non-critical care setting: An endocrine society clinical practice guideline: J Clin Endocrinol Metab, 2012; 97(1); 16-38
16.. Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN, Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD: J Clin Endocrinol Metab, 2011; 96(6); 1789-96
17.. Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients: Diabetol Metab Syndr, 2013; 5; 18
18.. Clore JN, Thurby-Hay L, Glucocorticoid-induced hyperglycemia: Endocr Pract, 2009; 15(5); 469-74
19.. Buren J, Lai YC, Lundgren M, Insulin action and signalling in fat and muscle from dexamethasone-treated rats: Arch Biochem Biophys, 2008; 474(1); 91-101
20.. Lambillotte C, Gilon P, Henquin JC, Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets: J Clin Invest, 1997; 99(3); 414-23
21.. Kishimoto M, Noda M, Verification of glycemic profiles using continuous glucose monitoring: Cases with steroid use, liver cirrhosis, enteral nutrition, or late dumping syndrome: J Med Invest, 2015; 62(1–2); 1-10
22.. Ruiz de Adana MS, Colomo N, Maldonado-Araque C, Randomized clinical trial of the efficacy and safety of insulin glargine vs. NPH insulin as basal insulin for the treatment of glucocorticoid induced hyperglycemia using continuous glucose monitoring in hospitalized patients with type 2 diabetes and respiratory disease: Diabetes Res Clin Pract, 2015; 110(2); 158-65
23.. Yata Y, Hosojima M, Kabasawa H, The assessment of the efficacy of dipeptidyl peptidase-4 inhibitors in patients with glucocorticoid-induced diabetes by continuous glucose monitoring: Intern Med, 2017; 56(19); 2555-62
24.. Tanaka K, Okada Y, Mori H, The effects of mitiglinide and repaglinide on postprandial hyperglycemia in patients undergoing methylprednisolone pulse therapy: Intern Med, 2018; 57(1); 65-70
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250