09 June 2021: Articles 
An Audiovisual 3D-Immersive Stimulation Program in Hemianopia Using a Connected Device
Unusual or unexpected effect of treatment
Monica Daibert-Nido1ABCDE, Yulia Pyatova1ABC, Kyle G. Cheung2AB, Arun Reginald34BCD, Eduardo Garcia-Giler25BC, Eric Bouffet4BCD, Samuel N. Markowitz12ABDF, Michael Reber2657ACDEFG*DOI: 10.12659/AJCR.931079
Am J Case Rep 2021; 22:e931079
Abstract
BACKGROUND: Homonymous hemianopia is a loss of conscious vision in one hemifield, strongly affecting everyday life. Audiovisual stimulation programs improve visual perception in the blind hemifield; however, they use large equipment operated in clinical settings. Such treatments require frequent visits at the clinic, hampering the patient’s adherence and compliance. In one hemianopia patient, we tested a 4-week dynamic audiovisual rehabilitation program in the stand-alone, remotely controlled, virtual-reality, head-mounted display Oculus Go and measured the effect on visual perception.
CASE REPORT: A 15-year-old Caucasian male was diagnosed with a right homonymous hemianopia with splitting of central fixation after a traumatic occipital contusion at age 7 months. Visual assessment showed impaired binocular contrast sensitivity and retinal sensitivity. Fixation stability and visual fields were strongly affected. After a 4-week audiovisual rehabilitation program, including 3 hours 20 minutes of stimulation, the contrast sensitivity, fixation stability, and paracentral visual perception were significantly enhanced, improving quality of life.
CONCLUSIONS: This pioneering work reports the use of virtual-reality in a head-mounted display to provide an audiovisual stimulation protocol for low-vision rehabilitation in a hemianopia patient. Real-time data recording and remote control of the stimulation program demonstrate that such rehabilitation treatment can be performed by the patient at home without interruption of care, decreasing the burden of disease. Beneficial effects on visual function were measured according to clinical guidelines of low-vision assessment. Improvement in visual function and quality of life challenge the prevailing belief that post-acute vision loss is both permanent and unchangeable.
Keywords: Contrast Sensitivity, Hemianopsia, Quality of Life, Rehabilitation, User-Computer Interface, Vision, Low, Adolescent, Infant, Visual Fields
Background
Homonymous hemianopia (HH) is a loss of conscious vision in the contra-lesional hemifield of both eyes. It is caused by post-chiasmatic injuries (eg, stroke, trauma, or tumors) affecting the optic tract, lateral geniculate nucleus, optic radiations, or primary visual cortex V1. HH is a debilitating condition. In addition to the inability to drive, read, or navigate, the loss of independence and inability to enjoy leisure activities can have significant emotional and social implications [1]. Patients present difficulties in detecting stimuli in the affected field region, showing impaired orientation and visual scanning [2] and a skewed auditory space, leading to imprecise sound localization [3]. These visual impairments translate into difficulties in distance vision, impacting orientation and mobility, and in near vision tasks such as reading [4]. Despite these sensory deficits, some patients retain the ability to integrate audiovisual stimuli in the affected visual field [5]. Spontaneous recovery of visual field deficits is common and well-demonstrated [1]; however, it is highly variable and is correlated with the severity of the injury [1]. Patients elaborate compensatory strategies, but there is no standardized rehabilitation treatment for homonymous patients [6]. Several visual rehabilitation protocols have been proposed with the primary objective of restoring visual fields [6–9]. Among them, audiovisual stimulations have been shown to improve oculomotor patterns and restore visual scanning toward the blind field [7,8,10–12], with beneficial effects lasting for several months [8]. This partial restoration of visual function is mediated by neuronal plasticity and network stimulation occurring within subcortical structures (superior colliculus, lateral geniculate nucleus and pulvinar) connected to extra-striate cortex and bypassing V1 [13–16]. Such plasticity, in the form of perceptual learning or long-term adaptation, is the consequence of repetitive stimuli performed during the audio-visual stimulation procedures [17–23]. One major drawback of current audiovisual stimulation protocols included in visual rehabilitation programs is the requirement of a clinical/laboratory setting to accommodate large screen displays and sound speakers, occupying substantial space [8,11,12,24]. For example, a 2 m wide×30 cm high ellipse-shaped apparatus has been used for audiovisual training in a laboratory setting [11,12,25]. Moreover, the patients must arrange frequent appointments at the clinic/ laboratory, generating logistic and financial burden and affecting adherence and compliance. Here, we tested the feasibility of a 4-week-long audiovisual stimulation protocol based on the 3D multiple object tracking (3D-MOT) paradigm in a hemianopia patient using an immersive virtual-reality (IVR) environment rendered in the stand-alone, remotely controlled, head-mounted display Oculus Go. As a secondary outcome, we measured the effects on visual function and functional vision. Although no improvement in field restoration were detected, we observed a significant increase in contrast sensitivity, fixation stability, and retinal sensitivity, which improved his quality of life.
Case Report
PATIENT INFORMATION:
A 15-year-old boy was referred to the Low-Vision service at the University Health Network Toronto Western Hospital. The patient had an accidental traumatic posterior occipital contusion in 2003 at age 7 months. He had multi-compartment hemorrhage, including a large left parietooccipital intraparenchymal hematoma, patchy diffusion restriction in the left temporal and parietal lobes, and bilateral frontal subdural effusions (Figure 1A, 1B). He was originally diagnosed with a right HH with splitting of central fixation at the Hospital for Sick Children in Toronto.
DIAGNOSTIC ASSESSMENT:
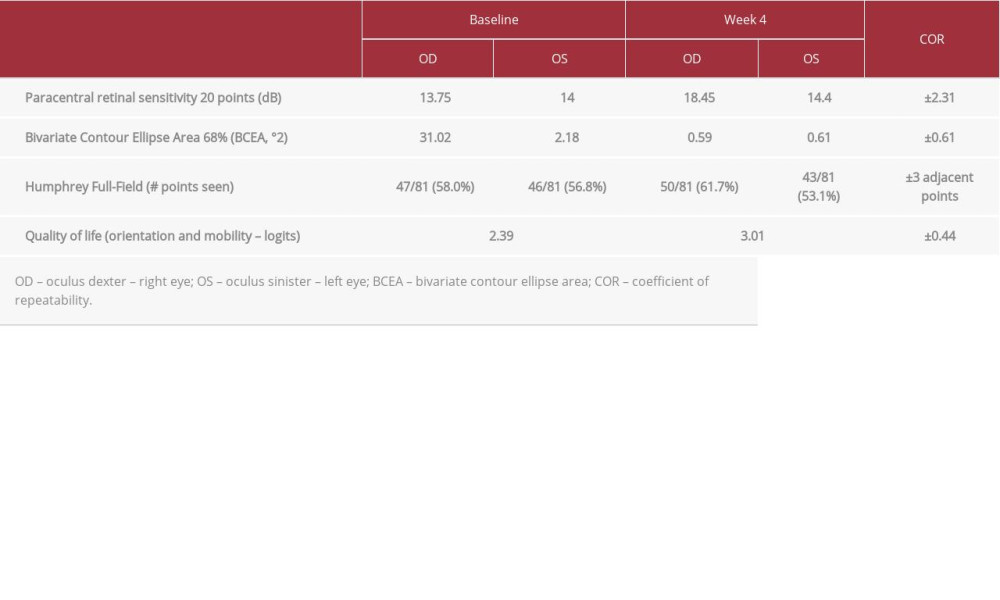
A low-vision assessment of the patient was performed by the ophthalmologists running the visual rehabilitation program, following previously described professional guidelines [26]. No visual neglect was demonstrated with the Star Cancellation tests [27]. Cognition tested with the Folstein Mini-Mental Status Examination [27] was normal. Best corrected visual acuity was 20/20 for each eye. The refractive error was −2.00+0.50x180 OD and −2.00 OS. Contrast sensitivity evaluated with the FACT test [27] showed more loss at the higher spatial frequencies for the left eye (OS) than the right eye (OD) (Figure 2A, 2B gray lines). Visual fields were mapped with the Nidek MP-1 Microperimetry C10-2 Program [27] (single-cross 2°, Goldman III stimulus). Both eyes show reduced paracentral retinal sensitivity of 13.75 dB and 14.00 dB for the right and left eye, respectively (Table 1). Fixation stability, as measured by Nidek MP-1 using the bivariate contour ellipse area 68% (BCEA 68%), was impaired in both eyes at 31.02°2 for OD and 2.18°2 for OS (Table 1). Visual field analysis, as measured by Humphrey field of view (Humphrey full-field 81, stimulus: III, white; background: 31.5 asb; central and peripheral reference: 34 dB) revealed 47/81 (58.0%) and 46/81 (56.8%) points seen for right and left eye, respectively (Table 1). The orientation and mobility subsection of the Veterans’ Affairs low-vision visual functioning questionnaire (LV-VFQ-48) [28,29] showed an ability index of 2.39 logits (Table 1).
THERAPEUTIC INTERVENTION:
Audiovisual stimulation in the head-mounted display Oculus Go took place once weekly over a 4-week span at the Low-Vision clinic at Toronto Western hospital. The patient was comfortably seated, wearing the Oculus Go adjusted to his face. Each stimulation session included 100 audiovisual trials divided in 10 sessions of 10 trials of 30 s each. Each trial consisted of identifying a moving target in a 3D-immersive environment on a black background (Figure 3). The target and visual distractors followed random linear movements across the visual field encompassing the blind field and bouncing on one another and on the walls of a virtual 3D cube (78° horizontal eccentricity, 50° vertical eccentricity) when collisions occurred. The target (a sphere of 1.57° visual angle, 81 cd/m2 luminosity) could be tracked among 8 distractors using both vision and audition when correlated spatial sound (60 Hz, 50 dB) accompanied the moving target. Correlated sound and target image are spatially and temporally readjusted every 16 ms, below the temporal timeframe (100 ms) required for multisensory response enhancement [19,22]. After 30 seconds of movement, the spheres stopped, and the patient had to select the cued target among the distractors using a hand-guided laser pointer. A correct selection was recorded as a positive hit. Speed of the spheres were 82.5°/s for week 1 and 2 and 90°/s for week 3 and 4. Visual assessment at 4 weeks was performed at the Low-Vision clinic at Toronto Western Hospital and was compared to baseline using the same methods and procedures described in diagnosis assessment. The performance of the patient (speed, positive hits) during the IVR stimulation was measured and recorded in real-time from another location at the hospital through Wi-Fi connectivity. Modifications to the audiovisual stimulation protocol within the Oculus Go were performed remotely though wi-fi using a dedicated web interface.
FOLLOW-UP AND OUTCOMES:
After the visual rehabilitation program, at week 4, contrast sensitivity measured with the FACT test showed significant improvements due to the treatment for both eyes (Figure 2A, 2B, coefficients of repeatability – COR=±0.24 log CS [30]). Paracentral retinal sensitivity, measured with the Nidek MP-1 Microperimetry C10-2 Program [26], showed a paracentral retinal sensitivity improvement in the right eye (ODbaseline=13.75 dB, ODtreatment=18.45 dB, COR=±2.31 dB [31], Table 1), with a strong effect of the treatment (percentage of data exceeding the median [32] – PEM=0.9). Fixation stability in both eyes was significantly enhanced after treatment, as measured by Nidek MP-1 (ODbaseline=31.02°2, ODtreatment=0.59°2, OSbaseline=2.18°2, OStreatment=0.67°2, COR=±0.61 [33], Table 1). Visual field scores measured by the Humphrey full-field 81 test did not indicate a significant improvement in either eye (Table 1). The orientation and mobility subsection of the LV-VFQ-48 quality of life questionnaire showed a significant improvement, from 2.39 logits at baseline to 3.01 logits after treatment (COR=±0.44 logits [29], Table 1).
Discussion
This report demonstrates the feasibility of a visual rehabilitation program using an IVR head-mounted display running an audiovisual stimulation program. The patient was able to follow the stimulation without experiencing adverse events related to the use of a virtual-reality head-mounted display [34,35]. Moreover, 400 audiovisual IVR tasks performed once a week for 4 weeks (for a total of 3 h 20 min of stimulation) significantly improved visual functions, including contrast sensitivity, fixation stability, and paracentral visual perception, positively impacting quality of life. Improvements in contrast sensitivity translate into the detection of texture on a background (eg, uneven grounds, curbside, stairs), improving mobility and orientation. Improvements in fixation stability and retinal sensitivity lead to a better visual acuity and reading capacity [36,37]. These results are unlikely to be due to a learning effect, as baseline and after-treatment testing at the clinic were separated by 4 weeks. Others have shown a learning effect lasting for up to 1 week between tests [38]. Monocular measures of contrast sensitivity and fixation stability indicated a significant effect of the audiovisual stimulation program on both eyes, whereas paracentral retinal sensitivity, also measured monocularly, showed a significant improvement for the right eye but not for the left eye. Contrast sensitivity, fixation stability, and retinal sensitivity measurements are not affected by ocular dominance [39,40]. One hypothesis for such a discrepancy in paracentral retinal sensitivity between the left and the right eye is that the audiovisual stimulation program was performed binocularly, which may have favored the dominant eye [41],although why this would have affected only paracentral sensitivity and not contrast sensitivity or fixation stability is unknown. No significant effect on visual field detection was observed in Humphrey full-field 81 analysis of the 3 h 20 min of audiovisual stimulation, suggesting that this duration is not enough to lead to detectable field improvements. This is line with previous work, indicating that an enhancement in visual field detection in the blind hemifield was observed after 40 h of audiovisual training, although using a different setup and device [11]. Here, positive effects on contrast sensitivity and fixation stability after only 3 h 20 min suggest that high-contrast dynamic stimulation procedures are efficient on these particular features but not on field restitution within that stimulation duration. Longer and more frequent stimulation sessions might be required to achieve more significant field restitution. Other visual function metrics will be assessed in the future. Particularly, we will measure the field of view using the Esterman binocular analysis [42], allowing us to test the patient’s vision in a 160° horizontal span and 100° vertical span (40° upper field and 60° lower field). This binocular test is a relevant readout to measure the potential improvement of peripheral vision in hemianopia patients after an audiovisual IVR stimulation program. Other metrics, generally used in low-vision rehabilitation procedures [26], will include near and far best corrected visual acuity (BCVA), reading speed using the MNREAD test [43], and the full Low-vision-Visual function Questionnaire (48 items) [28]. Visual detection will also be assessed using the useful field of view test [44]. Altogether, these tests will provide a broader appreciation of the patient’s visual function and functional vision.
Over the last 15 years, work in animal models and patients indicates that audiovisual stimuli, during which punctate auditory and flashed visual stimuli are spatially and temporally correlated, improve visual perception in hemianopia [5–12,19–23]. Here, we developed a portable, stand-alone and dynamic version of an audiovisual stimulation program, in which the moving cued target is continuously associated, spatially and temporally, with a moving sound. The correlated sound to the cued target is used as a lure to stimulate visual tracking and spatial localization, knowing that hemianopia patients often present impaired sound localization [3]. Our dynamic audiovisual stimulation procedure mimics more complex dynamical situations where individuals apprehend their environment using both visual and auditory information, therefore increasing the ecological validity of the rehabilitation.
Virtual-reality systems and stimulation show limited tolerance in individuals [45]. A correlation between sickness severity and exposure time (typically above 10 min) has been shown, and speed of motion, age, and gender all affect tolerability [45]. In this case report, the patient did not show any symptoms or adverse effects, considering that the sessions corresponded to 5 min of continuous stimulation, below the threshold of 10 min. In future studies, symptoms of virtual-reality stimulation will be assessed using the Virtual Reality Induced Symptoms and Effects (VRISE), a tool developed specifically to evaluate the adverse effects of VR stimulation [35].
Conclusions
Here, we obtained promising results demonstrating that a total of 3 h 20 min of audiovisual IVR stimulation procedure is sufficient to improve visual function and quality of life, with no significant field restitution, in a hemianopia teenage patient. Such results challenge the prevailing view that post-acute vision loss is both permanent and unchangeable. This work also demonstrates that dynamic audiovisual stimulation paradigms can be implemented in a stand-alone, remotely controlled, and user-friendly head-mounted device. Although this patient followed the rehabilitation program at the clinic, the real-time data recording and the control of the device/program were performed remotely from another location in the hospital, indicating that such stimulation protocol could be performed by the patient at home, thereby potentially decreasing the burden of disease and without interruption of care (particularly in pandemic-related restrictions). Further investigations will be undertaken with a longer training protocol (>6 weeks) with shorter sessions (<15 min) at higher frequency (>3 times/week) performed at home to evaluate the benefits of the audiovisual IVR stimulation program on visual field restitution.
Figures
References:
1.. Goodwin D, Homonymous hemianopia: challenges and solutions: Clin Ophthalmol Auckl NZ, 2014; 8; 1919-27
2.. Zihl J, Visual scanning behavior in patients with homonymous hemianopia: Neuropsychologia, 1995; 33(3); 287-303
3.. Lewald J, Peters S, Tegenthoff M, Hausmann M, Distortion of auditory space in hemianopia: Eur J Neurosci, 2009; 30(7); 1401-11
4.. de Haan GA, Heutink J, Melis-Dankers BJM, Difficulties in daily life reported by patients with homonymous visual field defects: J Neuroophthalmol, 2015; 35(3); 259-64
5.. Leo F, Bolognini N, Passamonti C, Cross-modal localization in hemianopia: New insights on multisensory integration: Brain, 2008; 131(3); 855-65
6.. Frolov A, Feuerstein J, Subramanian PS, Homonymous hemianopia and vision restoration therapy: Neurol Clin, 2017; 35(1); 29-43
7.. Grasso PA, Làdavas E, Bertini C, Compensatory recovery after multisensory stimulation in hemianopic patients: Behavioral and neurophysiological components: Front Syst Neurosci, 2016; 10; 45
8.. Dundon NM, Bertini C, Làdavas E, Visual rehabilitation: visual scanning, multisensory stimulation and vision restoration trainings: Front Behav Neurosci, 2015; 9; 192
9.. Sabel BA, Henrich-Noack P, Fedorov A, Gall C, Vision restoration after brain and retina damage: The “residual vision activation theory.”: Prog Brain Res, 2011; 192; 199-262
10.. Frassinetti F, Bolognini N, Làdavas E, Enhancement of visual perception by crossmodal visuo-auditory interaction: Exp Brain Res, 2002; 147(3); 332-43
11.. Bolognini N, Rasi F, Coccia M, Làdavas E, Visual search improvement in hemianopic patients after audio-visual stimulation: Brain J Neurol, 2005; 128(Pt 12); 2830-42
12.. Passamonti C, Bertini C, Làdavas E, Audio-visual stimulation improves oculomotor patterns in patients with hemianopia: Neuropsychologia, 2009; 47(2); 546-55
13.. Ajina S, Bridge H, Subcortical pathways to extrastriate visual cortex underlie residual vision following bilateral damage to V1: Neuropsychologia, 2019; 128; 140-49
14.. Ajina S, Pestilli F, Rokem A, Human blindsight is mediated by an intact geniculo-extrastriate pathway: eLife, 2015; 4; e08935
15.. Ajina S, Bridge H, Blindsight relies on a functional connection between hMT+ and the lateral geniculate nucleus, not the pulvinar: PLoS Biol, 2018; 16(7); e2005769
16.. Tamietto M, Morrone MC, Visual plasticity: blindsight bridges anatomy and function in the visual system: Curr Biol, 2016; 26(2); R70-73
17.. Huxlin KR, Martin T, Kelly K, Perceptual relearning of complex visual motion after V1 damage in humans: J Neurosci, 2009; 29(13); 3981-91
18.. Sagi D, Perceptual learning in Vision Research: Vision Res, 2011; 51(13); 1552-66
19.. Miller RL, Pluta SR, Stein BE, Rowland BA, Relative unisensory strength and timing predict their multisensory product: J Neurosci, 2015; 35(13); 5213-20
20.. Dakos AS, Jiang H, Stein BE, Rowland BA, Using the principles of multisensory integration to reverse hemianopia: Cereb Cortex, 2020; 30(4); 2030-41
21.. Jiang H, Stein BE, McHaffie JG, Multisensory training reverses midbrain lesion-induced changes and ameliorates haemianopia: Nat Commun, 2015; 6; 7263
22.. Meredith MA, Nemitz JW, Stein BE, Determinants of multisensory integration in superior colliculus neurons. I. Temporal factors: J Neurosci, 1987; 7(10); 3215-29
23.. Stein BE, Rowland BA, Using superior colliculus principles of multisensory integration to reverse hemianopia: Neuropsychologia, 2020; 141; 107413
24.. Targher S, Occelli V, Zampini M, Audiovisual integration in low vision individuals: Neuropsychologia, 2012; 50(5); 576-82
25.. Frassinetti F, Bolognini N, Bottari D, Audiovisual integration in patients with visual deficit: J Cogn Neurosci, 2005; 17(9); 1442-52
26.. Markowitz SN, Principles of modern low vision rehabilitation: Can J Ophthalmol, 2006; 41(3); 289-312
27.. Markowitz SN, A practice template for low-vision rehabilitation: Can J Ophthalmol, 2009; 44(5); 610
28.. Stelmack JA, Szlyk JP, Stelmack TR, Psychometric properties of the Veterans Affairs Low-Vision Visual Functioning Questionnaire: Invest Ophthalmol Vis Sci, 2004; 45(11); 3919-28
29.. Stelmack JA, Szlyk JP, Stelmack TR, Measuring outcomes of vision rehabilitation with the Veterans Affairs Low Vision Visual Functioning Questionnaire: Invest Ophthalmol Vis Sci Aug, 2006; 47(8); 3253-61
30.. Schilling T, Ohlendorf A, Leube A, Wahl S, TuebingenCSTest – a useful method to assess the contrast sensitivity function: Biomed Opt Express, 2017; 8(3); 1477-87
31.. Chen FK, Patel PJ, Xing W, Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease: Invest Ophthalmol Vis Sci, 2009; 50(7); 3464-72
32.. Lenz AS, Calculating effect size in single-case research: A comparison of nonoverlap methods: Meas Eval Couns Dev, 2012; 46(1); 64-73
33.. Chen FK, Patel PJ, Xing W, Intrasession repeatability of fixation stability assessment with the Nidek MP-1: Optom Vis Sci, 2011; 88(6); 742-50
34.. Kim HK, Park J, Choi Y, Choe M, Virtual reality sickness questionnaire (VRSQ): Motion sickness measurement index in a virtual reality environment: Appl Ergon, 2018; 69; 66-73
35.. Kourtesis P, Collina S, Doumas LAA, MacPherson SE, Validation of the Virtual Reality Neuroscience Questionnaire: Maximum duration of immersive virtual reality sessions without the presence of pertinent adverse symptomatology: Front Hum Neurosci, 2019; 13; 417
36.. Meyniel C, Bodaghi B, Robert P-Y, Revisiting vision rehabilitation: Front Syst Neurosci, 2017; 11; 82
37.. Mandelcorn MS, Podbielski DW, Mandelcorn ED, Fixation stability as a goal in the treatment of macular disease: Can J Ophthalmol J, 2013; 48(5); 364-67
38.. Acton JH, Bartlett NS, Greenstein VC, Comparing the Nidek MP-1 and humphrey field analyzer in normal subjects: Optom Vis Sci, 2011; 88(11); 1288-97
39.. Spry PGD, Furber JE, Harrad RA, The effect of ocular dominance on visual field testing: Optom Vis Sci, 2002; 79(2); 93-97
40.. Hirasawa K, Kobayashi K, Shibamoto A, Variability in monocular and binocular fixation during standard automated perimetry: PLoS One, 2018; 13(11); e0207517
41.. Shneor E, Hochstein S, Effects of eye dominance in visual perception: Int Congr Ser, 2005; 1282; 719-23
42.. Esterman B, Functional scoring of the binocular field: Ophthalmology, 1982; 89(11); 1226-34
43.. Legge GE, Ross JA, Luebker A, LaMay JM, Psychophysics of reading. VIII. The Minnesota Low-Vision Reading Test: Optom Vis Sci, 1989; 66(12); 843-53
44.. Woutersen K, Geuzebroek AC, van den Berg AV, Goossens J, Useful field of view performance in the intact visual field of hemianopia patients: Invest Ophthalmol Vis Sci, 2020; 61(5); 43
45.. Saredakis D, Szpak A, Birckhead B, Factors associated with virtual reality sickness in head-mounted displays: A systematic review and meta-analysis: Front Hum Neurosci, 2020; 14; 96
Figures
In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250