12 June 2021: Articles 
Aortic Angiosarcoma in Association with Endovascular Aneurysm Repair: Case Report and Review of the Literature
Rare disease
Kaori Takamura1ABCDEFG*, Hiroshi Kobayashi2ABCDEF, Brian P. Rubin3DEF, Shuhei Kondo1BCDF, Fuyuki Asami4BCD, Ryuji Aoyagi5BCD, Yoichi Ajioka1DGDOI: 10.12659/AJCR.931740
Am J Case Rep 2021; 22:e931740
Abstract
BACKGROUND: Primary aortic sarcoma often poses diagnostic challenges for pathologists and clinicians because of a very low incidence and controversy over nomenclature and definition. We report a case of aortic angiosarcoma in association with a graft. We also conducted a clinicopathological review of cases of primary aortic sarcomas associated with implanted grafts.
CASE REPORT: The patient was an 82-year-old woman. She underwent thoracic endovascular aneurysm repair (TEVAR) at age 78 because of an aneurysm in the descending aorta. Approximately 4 years after the TEVAR, computed tomography revealed a type II endoleak and expansion of the aneurysm. Her c-reactive protein level rose to 34 mg/dL, and Ga scintigraphy showed 67Ga accumulation at the aneurysm. She had fever up to 39°C, and a stent graft infection was suspected. Despite administration of antibiotics, her condition deteriorated, and she died. Postmortem examination identified epithelioid aortic angiosarcoma at the aorta with aneurysm repair and the graft, and the aortic angiosarcoma invaded the left lower lobe of the lung.
CONCLUSIONS: Our clinicopathological review revealed that the proper clinical diagnosis was very difficult owing to confusion of aortic sarcoma after the implantation with the infected graft, atypical endoleak, or pseudoaneurysm. The histological diagnosis was ambiguous because immunohistochemical and genetic studies were not adequately conducted. Overall prognosis of aortic sarcoma is poor as most patients die within a year, with no effective treatments. It is hoped that recent projects for genomic medicine will provide useful insights about the diagnosis and treatment of these cancers.
Keywords: Autopsy, Blood Vessel Prosthesis, Sarcoma, Aged, 80 and over, Aorta, Thoracic, Aortic Aneurysm, Thoracic, Blood Vessel Prosthesis Implantation, endovascular procedures, Hemangiosarcoma, Stents
Background
More than 160 cases of primary aortic sarcoma have been reported and two-thirds of them originated in the intima [1]. When sarcomas arise in the intima of great vessels of systemic and pulmonary circulation, they are conventionally classified as intimal sarcoma [2,3]. At least 70 cases of intimal sarcoma of the aorta have been reported in the English-language literature [2]. Serious diagnostic challenges for pathologists as well as clinicians still exist in the classification of primary sarcomas of great vessels owing to their very low incidence and controversy over nomenclature and definition [4–6]. Meanwhile, aortic sarcoma is rarely associated with implanted aortic grafts used in repair of aortic aneurysms. Over 20 cases have been reported, according to a search of the Medline database (Tables 1, 2) [7–26].
In this paper, we report a case of aortic angiosarcoma in association with an aortic graft that was recognized in the postmortem examination. We also conducted a comprehensive, clinicopathological review of the cases that have been reported as primary aortic sarcomas associated with implanted grafts. In the discussion, we address several issues related to aortic sarcomas, such as histological classification, clinicopathologic features, and updated genomic information.
Case Report
CLINICAL SUMMARY:
The patient was an 82-year-old woman. Her past medical history included tuberculosis at age 20, appendicitis at age 30, asthma at age 50, and acute cholecystitis at age 79. She had been on hemodialysis for 20 years for renal failure, likely caused by glomerulonephritis at age 37. She was found to have mitral valve regurgitation and low cardiac function at age 72. She complained of chest pain at age 76. Computed tomography (CT) identified an aortic aneurysm in the thoracoabdominal aorta. She underwent aortic replacement with a prosthetic graft. Another aneurysm of 6.5 cm in diameter was identified in the thoracic descending aorta at age 78. The aneurysm was repaired with thoracic endovascular aneurysm repair (TEVAR). Approximately 4 years after the TEVAR and 2 months before death, a type II endoleak and increase of the aneurysm diameter by 1 cm were identified by CT (Figure 1). C-reactive protein (CRP) was 1 to 2 mg/dL at the time of the TEVAR and showed a gradual increase: 5 to 6 mg/dL, 13 mg/dL, and 34 mg/dL at 8 months, 2 months, and 1 month before death, respectively. The patient was hospitalized for intensive examination and treatment 40 days prior to death. She had an intermittent fever of up to 39°C during hospitalization. Although CRP levels remained high, procalcitonin was 0.73 ng/mL and 1.73 ng/mL (cut off value was 0.3 ng/mL) 1 month and 2 days before death, respectively. βD-glucan was within normal limits and blood cultures were negative. Nevertheless, culture of pus from her oral cavity demonstrated Candida albicans 1 week prior to death. No vegetation was seen on the heart valves by echocardiography. Thrombus in the right pulmonary artery was identified by CT. Ga scintigraphy indicated 67Ga accumulation at the repaired aorta with the stent graft. Antibiotics were administered on suspicion of stent graft infection. However, the patient’s physiological state deteriorated, and she died. Postmortem examination was performed.
PATHOLOGICAL FINDINGS:
An autopsy was performed to confirm (1) the stent graft infection and its cause and (2) the cause of death. Macroscopically, there was a hemorrhagic, 10×6.5×2-cm, crescent-shaped mass with brownish color along the aortic wall. The tumor was compressed and adhered to the mediastinal pleura of the left lower lobe of the lung. A thin layer of coagulated blood and fibrin exudate appeared to cover the intimal surface of the mass (Figure 2A). Histologically, pleomorphic atypical cells proliferated along hemorrhagic spaces or slit-like channels. The neo-plastic cells were located on the intima of the aneurysm wall and invaded the aortic tunica media and externa and partially into pulmonary tissue beyond the pleural elastic membrane (Figure 2B). The tumor cells were round or oval in shape and had abundant eosinophilic cytoplasm with large, vesicular nuclei and prominent nucleoli (Figure 3A, 3B). The neoplasm was extensively necrotic with many neutrophils and macrophages.
Immunohistochemically, almost all the neoplastic cells strongly expressed vimentin and CD31, and most of them positively expressed ERG, Factor VIII, and AE1/AE3 (Figure 3C, 3D), but they were negative for CD34 and TTF-1. The neoplasm was diagnosed as epithelioid aortic angiosarcoma of the aorta at the stent graft. VEGFR3 and CDK4 were weakly positive, and MDM2 and C-MYC were completely negative.
No metastasis to other organs was found. Mild to moderate bronchopneumonia was found in the bilateral lungs. Microscopic abscesses were seen in the lung, heart, and liver. Moreover, small amounts of
Discussion
Sarcomas of the great vessels of the systemic and pulmonary circulation usually arise in the intima and exhibit primarily intraluminal growth [2,3]. Accordingly, the term intimal sarcoma is often employed for these tumors. The definition by the AFIP acknowledges tumors with any specific types of histology as intimal sarcomas when they arise from the intima of the great vessels [2]. However, the WHO in 2019 defined intimal sarcomas as morphologically non-distinctive, poorly differentiated malignant mesenchymal tumors with or without heterologous elements such as neoplastic cartilage, tumor osteoid, or focal rhabdomyosarcomatous or angiosarcomatous features [3]. The WHO further maintains that intimal sarcoma has strong nuclear expression of MDM2 protein and high-level amplification of
Regarding aortic intimal sarcoma, at least 70 cases have been reported, according to the AFIP [2]. Over half of the cases were classified as aortic angiosarcoma, followed by poorly differentiated sarcomas of fibroblastic or myofibroblastic differentiation. Rustoven et al have done a systematic review of aortic sarcomas through a search of the Medline database [1]. Among the 165 cases they identified, the most common site of origin was the aortic intima (110, 66.7%). Undifferentiated tumor histology was most common (65, 39.4%), followed by vascular (61, 37%) and smooth muscle (22, 13.3%). However, most of the cases have been reported in the radiologic or surgical literature without thorough immunohistochemical or molecular analysis. There are only a few reports of immunohisto-chemical studies with MDM2 and CDK4 and analysis by FISH of
Aortic sarcoma in association with an implanted aortic graft is very rare. To the best of our knowledge, 23 cases, including the present case, have been reported, as listed in Tables 1 and 2. The patient ages ranged from 48 to 86 years with an average of 66.6 years. Female to male ratio is 2: 21. The site of the repaired aneurysm was thoracic aorta in 7 cases and abdominal aorta in 16 cases. Patients most frequently reported a wide variety of nonspecific symptoms such as general fatigue, abdominal pain, weight loss, and fever. The proper clinical diagnosis was very difficult owing to frequent confusion of aortic sarcoma after implantation of graft with the infected graft (10/23), atypical endoleak (9/23), or pseudoaneurysm (6/23) while mass (4/23) was suspected on imaging. Although all of the conditions can have overlapping features with sarcoma, they are more likely to be suspected than sarcoma since they are clinically much more common [23]. However, no patients who were suspected of having an infected graft finally demonstrated positive blood or tissue culture or evidence of purulence. Accordingly, clinical suspicion of infection may have been attributable to neoplastic fever. Over a dozen papers have indicated that neoplastic fever ranges from 7.0% to 35.4% in frequency among patients with malignancy [31]. Nakamura et al reported that neoplastic fever was observed in 11 (5.6%) of 195 patients with bone and soft tissue sarcomas [32]. Neoplastic fever in the setting of aortic sarcoma requires further investigation.
It is reported that 10% of patients with aortic intimal sarcoma present with an abdominal aortic aneurysm [2]. Another study reported that 26.7% of 165 aortic sarcomas were misattributed to aneurysm/pseudoaneurysm [1]. Consequently, diagnosis of aortic sarcomas associated with aneurysm repair is difficult. As shown in Tables 1 and 2, the time to development or detection of intimal aortic sarcoma after surgery ranges from 4 months to 17 years. The time of the two different surgical procedures seems to be similar in length. There is no certain evidence of the tumor growth rate in the aorta, where neoplastic conditions are exceedingly rare. However, the tumors that developed within 6 months after surgery most likely existed in the treated aorta because all were large in size at the time of detection; one that arose at 4 months was 7.0 cm, another that was found at 6 months was 7.3 cm, and the other case that was identified at 6 months was described as a large tumor.
To repair an aortic aneurysm, open surgery, namely aortic replacement with a prosthetic graft, was the most common technique until early 2000. A minimally invasive surgery, endovascular aneurysm repair, was introduced three decades ago and is now the dominant treatment, as demonstrated in Table 1. A case series with aortic intimal sarcoma [5] and another with aortic sarcoma [1] showed similar frequencies of sarcomas in association with synthetic prostheses, with frequencies of 4.5% and 6.7%, respectively. These numbers suggest that there did not seem to be a strong correlation between these sarcomas and the synthetic graft. Some reports indicated that the association could be coincidental because there were very small number of cases with tumors, in comparison with the large number of cases with therapeutic procedures performed [5,23].
Meanwhile, the graft can increase the likelihood of malignancy secondary to chronic injury/inflammation or some other mechanism [5]. Additionally, in Table 2, aortic angiosarcoma is the most common (75% of cases) type of sarcoma associated with the implanted graft, compared with other types of sarcoma, such as undifferentiated sarcomas, although immunohistochemistry analysis was not performed in some cases, limiting the conclusions that can be made. Only a few genomic studies of aortic angiosarcoma demonstrated that some distinct molecular patterns of aortic angiosarcoma occurred at distinct sites: high-level
Prognosis of aortic sarcoma in association with an implanted graft is poor, as most patients died within a year after diagnosis, as shown in Table 2. Since the clinical and/or histological diagnoses can be extremely difficult, the tumor can rapidly progress before a definitive diagnosis is made. Consequently, a novel diagnostic method to detect the tumor in an early and precise manner would be useful. There are recent studies on the potential role of microRNAs in diagnosing sarcomas [38,39]. Rare cancers like aortic angiosarcoma are a significant unmet clinical need because they have limited large-scale clinical and genomic studies. To overcome these challenges, the Angiosarcoma Project was initiated in the United States and Canada, generating genomic data [34]. In Japan, the Master Key Project started in 2017 at the National Cancer Center [40] to promote genomic medicine in rare cancers. It is hoped that these dedicated projects will provide useful insights about the diagnosis and treatments of these rare cancers.
Conclusions
We reported a rare autopsy case of angiosarcoma that arose in the thoracic descending aorta that had been treated with a stent graft 4 years before. Since the patient’s main symptoms and clinical data were high fever, high CRP level, and type II endoleak, such nonspecific findings could not lead us to a sarcoma as an antemortem diagnosis. Reviewing this case and related literature, we believe that detailed clinicopathologic, immunohistochemical, and molecular analyses, as well as accumulation of accurate data, are important to develop a novel diagnostic and treatment method to detect such rare tumors.
Figures
References:
1.. Rusthoven CG, Liu AK, Bui MM, Sarcomas of the aorta: A systemic review and pooled analysis of published reports: Ann Vasc Surg, 2014; 28; 515-25
2.. Burke A, Tabora FR, Maleszewski JJ: Tumors of the great vessels In: AFIP Atlas of Tumor Pathology Tumors of the heart and the great vessels, 4th Series, Fasicle 22, 2015, Washington, DC, American Registry of Pathology
3.. Bode-Lesniewska B, Debiec-Rychter M, Intimal sarcoma: WHO classifications of tumours of soft tissue and bone, 2019; 315-17, Lyon, International Agency for Research on Cancer
4.. Fatima J, Duncan AA, Maleszewski JJ, Primary angiosarcoma of the aorta, great vessels, and the heart: J Vasc Surg, 2013; 57; 756-64
5.. Staats P, Tavora F, Burke AP, Intimal sarcomas of the aorta and iliofemoral arteries: A clinicopathological study of 26 cases: Pathology, 2014; 46; 596-603
6.. Restrepo CS, Betancourt SL, Martinez-Jimenez S, Gutirrez FR, Tumors of the pulmonary artery and veins: Semin Ultrasound CT MRI, 2012; 33; 580-90
7.. O’Connell TX, Fee HJ, Golding A, Sarcoma associated with Dacron prosthetic material. Case Report and review of the literature: J Thorac Cardiovasc Surg, 1976; 72; 94-96
8.. Weinberg DS, Manini BS, Primary sarcoma of the aorta associated with a vascular prosthesis: A case report: Cancer, 1980; 46; 398-402
9.. Fehrenbacher JW, Bowers W, Strate R, Angiosarcoma of the aorta associated with a Dacron graft: Ann Thorac Surg, 1981; 32; 297-301
10.. Paterson HS, Meredith DJ, Craddock DR, Malignant fibrous histiocytoma associated with a Dacron vascular prosthesis: Ann Thorac Surg, 1989; 47; 772-74
11.. Weiss WM, Riles TS, Gouge TH, Angiosarcoma at the site of a Dacron vascular prosthesis: A case report and literature review: J Vasc Surg, 1991; 14; 87-91
12.. Fyfe BS, Quintana CS, Kaneko M, Aortic sarcoma four years after Dacron graft insertion: Ann Thorac Surg, 1994; 58; 1752-54
13.. Ben-Izhak O, Vlodavsky E, Ofer A, Epithelioid angiosarcoma associated with a Dacron vascular graft: Am J Surg Pathol, 1999; 23; 1418-22
14.. Okada M, Takeuchi E, Mori Y, An autopsy case of angiosarcoma arising around a woven Dacron prosthesis after a Cabrol operation: J Thorac Cardiovasc Surg, 2004; 127; 1843-45
15.. Umscheid TW, Rouhani G, Morlang T, Hemangiosarcoma after endovascular aortic aneurysm repair: J Endvasc Ther, 2007; 14; 101-5
16.. Alexander JJ, Moawad J, Cai D, Primary intimal sarcoma of the aorta associated with a Dacron graft and resulting in arterial rupture: Vasc Endvasc Surg, 2007; 40; 509-15
17.. Garg N, Lewis MA, Maleszewski JJ, Intimal sarcoma in an inflammatory aneurysm after endovascular aneurysm repair: J Vasc Surg, 2012; 55; 1134-37
18.. Schmehl J, Scharpf M, Brechtel K, Epithelioid angiosarcoma with metastatic disease after endovascular therapy of abdominal aortic aneurysm: Cardiovasc Intervent Radiol, 2012; 35; 190-93
19.. Stewart B, Manglik N, Zhao B, Aortic intimal sarcoma: Report of two cases with immunohistochemical analysis for pathogenesis: Cardiovasc Pathol, 2013; 22; 351-56
20.. Fenton J, Veenstra M, Bove P, Angiosarcoma involving native abdominal aortic aneurysm sac after endograft repair: Ann Vasc Surg, 2014; 28; 490.e1-e4
21.. Kimura S, Yonekura R, Umesue M, Angiosarcoma mimicking an infected pseudoaneurysm after graft replacement: Ann Thorac Surg, 2015; 100; 1114
22.. Milite D, Pilon F, Ferrari A, Aortic epithelioid angiosarcoma after endovascular aneurysm repair: Ann Vasc Surg, 2016; 35; 207.e17-e21
23.. Kamran M, Fowler KJ, Mellnick VM, Multimodality imaging approach towards primary aortic sarcoma arising after endovascular abdominal aortic aneurysm repair: Case series report: Cardiovasc Intervent Radiol, 2016; 39; 940-47
24.. Whittington EA, Duncan LD, McNally MM, Pleomorphic undifferentiated aortic sarcoma presenting as persistent endoleak after endovascular aneurysm repair: J Vasc Surg Case Innov Techniq, 2019; 5; 294-97
25.. Yu PC, Aplin B, Reed AB, Angiosarcoma of the abdominal aorta after endovascular aneurysm repair: J Vasc Surg Case Innov Techniq, 2019; 5; 506-8
26.. Natsume K, Shiiya N, Tsuchida T, Intimal sarcoma in an ascending aortic Dacron graft mimicking a thrombus: Inter Cardiovasc Thorac Surg, 2019; 29; 983-85
27.. Neuville A, Collin F, Bruneval P, Parrens M, Intimal sarcoma is the most frequent primary cardiac sarcoma: Clinicopathologic and molecular retrospective analysis of 100 primary cardiac sarcomas: Am J Surg Pathol, 2014; 38; 461-69
28.. Agaimy A, Ben-Izak O, Lorey T, Angiosarcoma arising in association with vascular Dacron grafts and orthopedic joint prostheses: Clinicopathologic, immunohistochemical, and molecular study: Ann Diagn Pathol, 2016; 21; 21-28
29.. Tajima S, Takahashi Y, Takahashi T, Intimal sarcoma of the abdominal aorta with platelet-derived growth factor receptor α overexpression and amplification in mural invasive cells and pulmonary metastatic cells but not in intimal spreading cells: Pathol Int, 2015; 65; 426-31
30.. Kobayashi H, Kobayashi Y, Yuasa S, A case of undifferentiated sarcoma in the superior vena cava and bilateral cervical veins: Am J Case Rep, 2018; 19; 1507-14
31.. Wright WF, Auwaerter PG, Fever and fever of unknown origin: Review, recent advances, and lingering Dogma: Open Forum Infect Dis, 2020; 7; cfaa132
32.. Nakamura T, Matsumine A, Matsubara T, Neoplastic fever in patients with bone and soft tissue sarcoma: Mol Clin Oncol, 2016; 5; 631-34
33.. Huang SC, Zhang L, Sung YS, Recurrent CIC gene abnormalities in angiosarcomas: A molecular study of 120 cases with concurrent investigation of PLCG1, KDR, MYC, and FLT4 gene alterations: Am J Surg Pathol, 2016; 40; 645-55
34.. Painter CA, Jain E, Tomson BN, The angiosarcoma project: Enabling genomic and clinical discoveries in a rare cancer through patient-partnered research: Nat Med, 2020; 26; 181-87
35.. Bode-Lesniewska B, Zhao J, Biraima AM, Gains of 12q13-14 and over-expression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery: Virchow Arch, 2001; 438; 57-65
36.. Dewaele B, Floris G, Finalet-Ferreiro J, Coactivated platelet-derived growth factor receptor α and epidermal growth factor receptor are potential targets in intimal sarcoma: Cancer Res, 2010; 70; 7304-14
37.. Van Dievel J, Sciot R, Delcroix M, Single-center experience with intimal sarcoma, an ultra-orphan, commonly fatal mesenchymal malignancy: Oncol Res Treat, 2017; 40; 353-59
38.. Asano N, Matsuzaki J, Ichikawa M, A serum microRNA classifier for the diagnosis of sarcoma of various histological subtypes: Nat Commun, 2019; 10; 1299
39.. Smolle MA, Leithner A, Posch F, MicroRNAs in different histologies of soft tissue sarcomas: A comprehensive review: Int J Mol Sci, 2017; 18; 1960
40.. Okuma HS, Fujiwara Y, Have we found the key to unravel treatment development lags for rare cancers? Master Key Project: Clin Pharmacol Ther, 2019; 106; 491-92
Figures
Tables
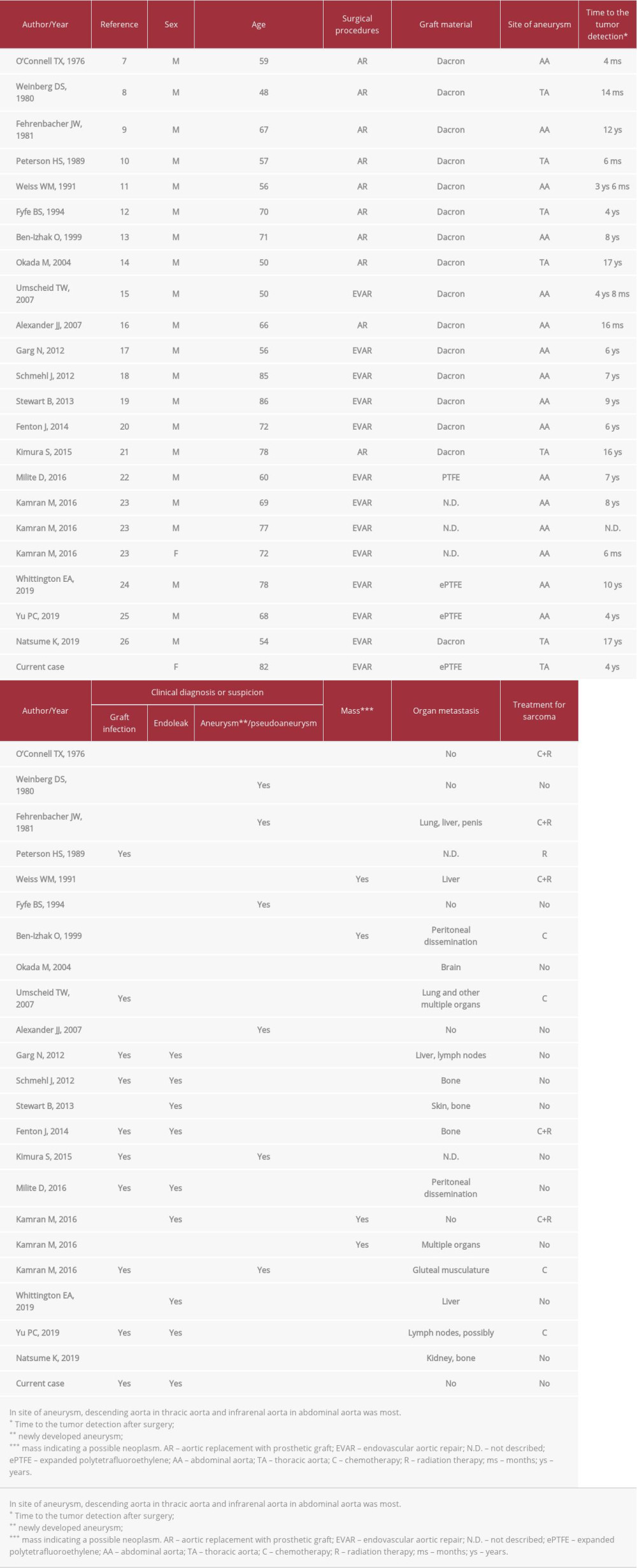 Table 1.. Clinical features of reported cases with aortic sarcoma associated with aortic prostheses.
Table 1.. Clinical features of reported cases with aortic sarcoma associated with aortic prostheses.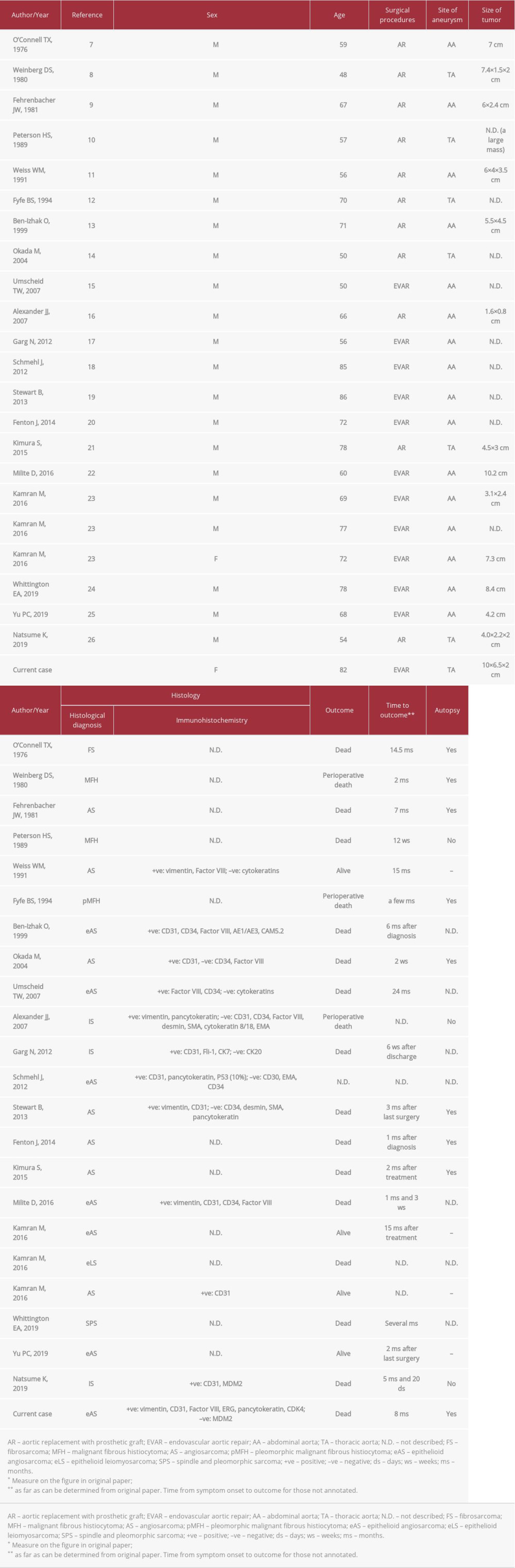 Table 2.. Clinicopathologic features of reported aortic angiosarcomas associated with aortic prostheses.
Table 2.. Clinicopathologic features of reported aortic angiosarcomas associated with aortic prostheses. Table 1.. Clinical features of reported cases with aortic sarcoma associated with aortic prostheses.
Table 1.. Clinical features of reported cases with aortic sarcoma associated with aortic prostheses. Table 2.. Clinicopathologic features of reported aortic angiosarcomas associated with aortic prostheses.
Table 2.. Clinicopathologic features of reported aortic angiosarcomas associated with aortic prostheses. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








