11 August 2021: Articles 
A 19-Year-Old Man with a History of Recreational Inhalation of Nitrous Oxide with Severe Peripheral Neuropathy and Central Pulmonary Embolism
Unusual clinical course, Challenging differential diagnosis
Oliver Buchhave Pedersen12CDEF, Anne-Mette Hvas13CDE, Erik Lerkevang GroveDOI: 10.12659/AJCR.931936
Am J Case Rep 2021; 22:e931936
Abstract
BACKGROUND: Recreational use of nitrous oxide (laughing gas) is a growing phenomenon among young people due to easy accessibility and a presumed innocent effect. However, complications have been reported, especially following high and long-term use, including nerve damage, spontaneous pneumo-mediastinum, myocardial infarction, and macrocytic anemia.
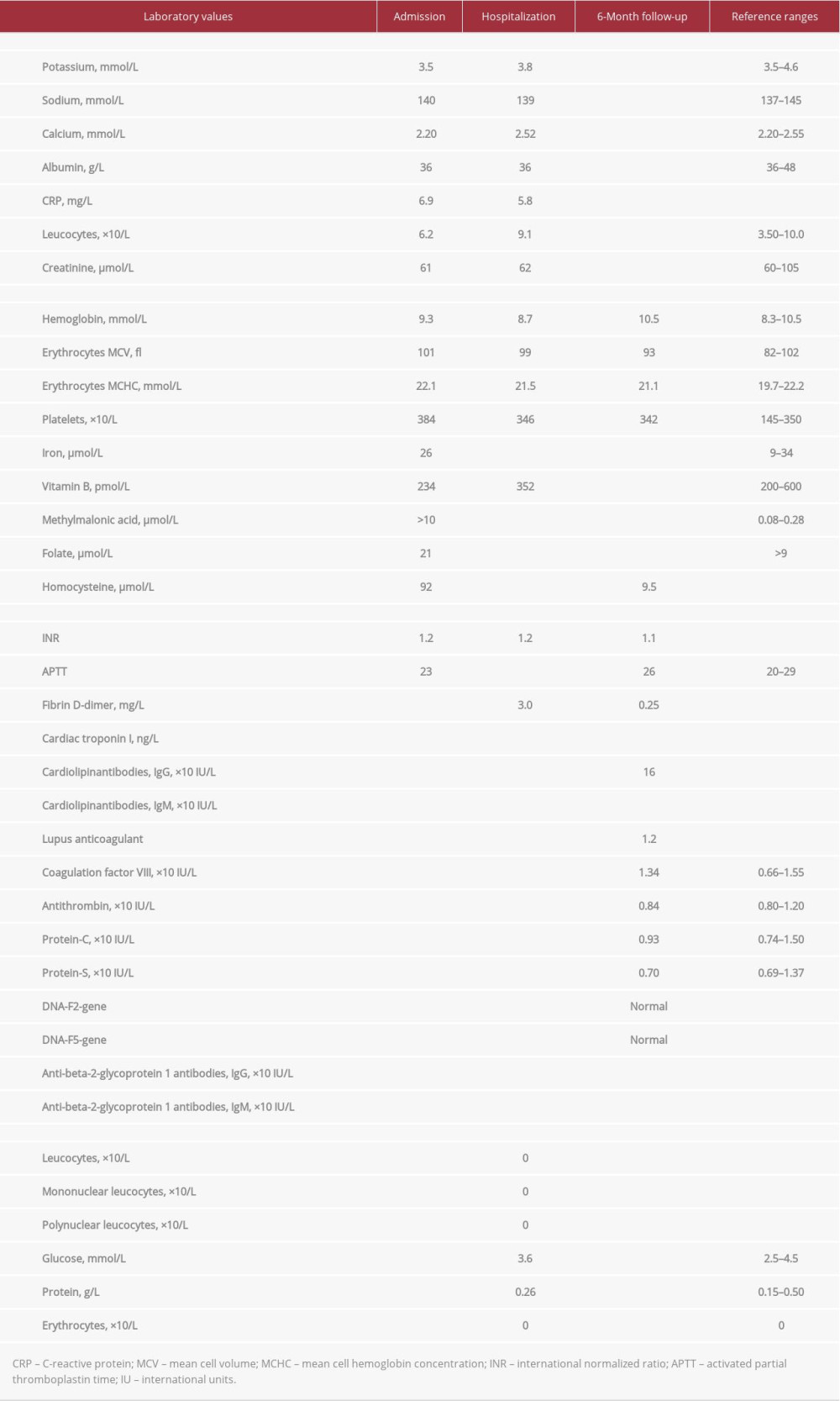
CASE REPORT: We report a case of a 19-year-old previously healthy man with occasional recreational use of nitrous oxide of up to 10 times within recent months, who presented with severe peripheral neuropathy. Laboratory examination revealed severely elevated homocysteine values of 92 µmol/L (reference range, <10 µmol/L), strongly elevated methylmalonic acid level of >10 µmol/L (range, 0.1-0.4 µmol/L), vitamin B₁₂ level of 234 pmol/L (range, 200-600 pmol/L), hemoglobin level of 9.3 mmol/L (range, 8.3-10.5 mmol/L), platelets of 384×10⁹/L (range, 145-350×10⁹/L), and leucocytes of 6.2×10⁹/L (range, 3.5-10.0×10⁹/L). Nitrous oxide can result in vitamin B₁₂ inactivation and nerve damage due to lack of myelination. During hospitalization, the patient had a bilateral central pulmonary embolism, probably caused by a combination of nitrous oxide abuse and some extent of immobilization. After 6 months of nitrous oxide cessation and treatment with B vitamins, the patient experienced almost no residual symptoms, and homocysteine and methylmalonic acid levels normalized.
CONCLUSIONS: Our case shows that even moderate recreational use of nitrous oxide can lead to severe peripheral neuropathy as well as increase the risk of thromboembolic complications. Especially young and previously healthy individuals presenting with unexplained neuropathy or thromboembolic events should therefore be asked about possible use of nitrous oxide.
Keywords: homocysteine, Nitrous Oxide, Peripheral Nervous System Diseases, Pulmonary Embolism, case reports, Adolescent, Vitamin B 12, Vitamin B 12 Deficiency, young adult
Background
Nitrous oxide has been used as an anesthetic for more than a century [1]. However, nitrous oxide in the form of so-called “laughing gas” is an increasingly used drug among young people [2]. This is owing to a presumed innocent effect combined with an easy and legal availability, as nitrous oxide can be easily obtained from, for example, whipped cream charging bottles [2]. Daily, large-scale (more than 200 cartridges), and long-term recreational use of nitrous oxide has been associated with nerve damage due to functional vitamin B12 deficiency [1,3,4]. Furthermore, various complications, including nerve damage, macrocytic anemia, thromboembolic phenomena, myocardial infarction, and spontaneous pneumo-mediastinum, have been associated with daily and long-term recreational use of nitrous oxide [5–11]. Deaths have also been described as a result of recreational use of nitrous oxide, presumably triggered by hypoxia [2,12].
This report is a case of a 19-year-old man with no preexisting cause of peripheral neuropathy but a recreational inhalation of nitrous oxide, who presented with peripheral neuropathy and had a pulmonary embolism during hospitalization.
Case Report
A 19-year-old man who was previously healthy without any medication or predisposition presented to the Emergency Department after falling down a staircase. He explained that, during the past 4 to 5 weeks, he had experienced increasing sensory disturbances in the hands and lower extremities and now experienced balance impairment and muscle weakness in the lower extremities. An extensive workup was performed, including neurological examination, lumbar puncture, electroneuronography, and magnetic resonance imaging (MRI) of the medulla totalis. The patient’s lumbar puncture and electroneuronography results were normal, and no definite pathological signal changes were recognized on the MRI scan (Figure 1). Furthermore, laboratory examination revealed highly elevated homocysteine values of 92 µmol/L (reference range, <10 µmol/L), strongly elevated methylmalonic acid level of >10 µmol/L (range, 0.08–0.28 µmol/L), normal folate level of 21 nmol/L (range, >9 nmol/L), normal vitamin B12 level of 234 pmol/L (range, 200–600 pmol/L), and a normal creatinine level of 61 µmol/L (range, 60–105 µmol/L) (Table 1). All other laboratory results were within the reference range (Table 1). Based on this extensive workup, the patient was diagnosed with severe sensorimotor neuropathy. The initial presumed diagnoses included Guillain-Barre syndrome, neurological infection, and other causes of neuropathy. However, based on the patient’s history and diagnostic workup, including laboratory results, it was concluded that the patient had sensorimotor neuropathy due to functional vitamin B12 deficiency. A potential underlying trigger was suggested to be moderate recreational use of nitrous oxide on various occasions, primarily on weekends. The patient claimed to had used nitrous oxide about twice per week within recent months (maximum of 50–75 cartridges per time), which the patient confirmed to several health professionals. The patient denied the use of other forms of recreational drugs. The patient received oral vitamin treatment with 1 mg once-daily vitamin B12, 5 mg once-daily folate (vitamin B9), and 300 mg once-daily thiamin (vitamin B1) and started physiotherapy treatment. The symptoms improved considerably during hospitalization, and after 14 days, the patient was ready for discharge for further outpatient treatment at a rehabilitation center. However, just before discharge, the patient suddenly developed severe non-radiating thoracic pain. The pain was worsened by deep inspiration. His vital signs were blood pressure of 112/64 mm Hg, pulse of 86 beats per min, and oxygen saturation of 98%. An acute computed tomography angiography of the aorta was performed on suspicion of aortic dissection. No dissection was found; however, bilateral central pulmonary embolism with signs of pulmonary infarction in both lower lobes with consolidated changes was found. Because the patient was completely hemodynamically stable and had a normal echocardiography, the pulmonary embolism was treated with an oral anticoagulant (20 mg once-daily rivaroxaban). After a few days of further hospitalization, the patient was discharged.
At clinical follow-up 6 months after hospitalization, only mild sensory-motor residual symptoms were present. The patient had no symptoms related to pulmonary embolism or anticoagulant treatment. A thrombophilia screening was performed, but all tests were normal, including no antiphospholipid antibodies or lupus anticoagulant, no genetic polymorphisms, and no lack of natural anticoagulants, (Table 1). In addition, the homocysteine level had been completely normalized (9.5 µmol/L) (Table 1). Based on these findings, the anticoagulant treatment was stopped.
Discussion
Our patient had both severe peripheral neuropathy and bilateral central pulmonary embolism, presumably due to functional vitamin B12 deficiency and increased level of homocysteine after moderate recreational use of nitrous oxide. In addition, after 6 months of total nitrous oxide cessation, the patient experienced almost no residual symptoms.
Peripheral neuropathy following recreational use of nitrous oxide has been reported previously, primarily in cases of long-term use [13–15]. Chronic use of nitrous oxide leads to functional vitamin B12 deficiency due to irreversible inactivation of vitamin B12 [2,16]. Active vitamin B12 is required for the conversion of methylmalonic acid and the degeneration of homocysteine to methionine, hence persistent inactivation of vitamin B12 by nitrous oxide results in elevated levels of methylmalonic acid and homocysteine [13,17] (Figure 2). As methionine is required for all methylations, vitamin B12 inactivation caused by the use of nitrous oxide can cause nerve damage due to lack of myelination of the nerve cell axons [13,18]. This mechanism is also a likely explanation for the peripheral neuropathy in our patient, as laboratory examinations at admission revealed elevated levels of methylmalonic acid and homocysteine, whereas plasma levels of vitamin B12 and folate as well as creatinine were normal. Although the patient’s vitamin B12 level was normal, the amount of cobalamin available for the cells can still be suboptimal and result in the experienced symptoms, as only the cobalamin bound to transcobalamin are available for circulation [19]. Unfortunately, transcobalamin was not measured in the patient. Furthermore, the MRI scan did not reveal any definite pathological findings. Following nitrous oxide cessation, the inactivation of vitamin B12 no longer persisted, leading to normalization of blood levels and restoration of nerve cell myelination. We believe that the rapid fall/normalization in homocysteine level supports the hypothesis that it is most likely the use of nitrous oxide causing the substantial increase in homocysteine level and the cessation causing the normalization.
Our patient had a pulmonary embolism 2 weeks after admission. Immobilization is a well-known risk factor for thromboembolic complications [20] and might be of importance regarding this case, as the patient primarily stayed in his own hospital room during hospitalization. However, the patient was not immobile and could still walk despite peripheral neuropathy. The use of nitrous oxide may also have contributed to the development of the pulmonary embolism. Accordingly, one case has previously been reported on pulmonary embolism following the recreational use of nitrous oxide, although this patient had long-term use [6]. Among potential reasons for the increased risk of thromboembolic complications is the highly increased level of homocysteine [6,21,22]. An increased level of homocysteine has been reported to have various effects regarding hemostasis including endothelium dysfunction, platelet activation, and impaired fibrinolysis, which can also increase the risk of myocardial infarction [23–25]. However, these consequences of increased homocysteine by nitrous oxide may not account for all effects of nitrous oxide on hemostasis. In a case report on a patient with an aortic arch thrombus following recreational use of nitrous oxide, the author suggested that since this thrombus occurred in an artery, a strong factor contributing to a hypercoagulable state must be present, and believed this factor to be nitrous oxide because the patient did not have any other major risk factors [26]. In addition, another case of a patient with isolated cortical vein thrombosis after long-term recreational use of nitrous oxide found a normal homocysteine level upon admission [27]. However, as our patient had a pulmonary embolism about 2 weeks after his last recreational use of nitrous oxide, the direct contributing effect of nitrous oxide on the development of pulmonary embolism was uncertain.
Conclusions
This report shows that even moderate recreational use of nitrous oxide can lead to severe peripheral neuropathy due to functional vitamin B12 deficiency and can be associated with pulmonary embolism, even in young and previously healthy individuals. Complete cessation of nitrous oxide use and treatment with relevant vitamins can lead to a rapid return to normal conditions. Due to an increasing consumption among young people, healthcare professionals should explore the possible recreational use of nitrous oxide in patients presenting with unexplained neuropathy or thromboembolic events. Furthermore, it is important that young people in particular are informed about this risk of adverse effects of the recreational use of nitrous oxide.
Figures
References:
1.. Emmanouil DE, Quock RM, Advances in understanding the actions of nitrous oxide: Anesth Prog, 2007; 54; 9-18
2.. van Amsterdam J, Nabben T, van den Brink W, Recreational nitrous oxide use: Prevalence and risks: Regul Toxicol Pharmacol, 2015; 73; 790-96
3.. Stockton L, Simonsen C, Seago S, Nitrous oxide-induced vitamin B12 deficiency: Proc (Bayl Univ Med Cent), 2017; 30; 171-72
4.. Samia AM, Nenow J, Price D, Subacute combined degeneration secondary to nitrous oxide abuse: quantification of use with patient follow-up: Cureus, 2020; 12; e11041
5.. Chanarin I, The effects of nitrous oxide on cobalamins, folates, and on related events: Crit Rev Toxicol, 1982; 10; 179-213
6.. Sun W, Liao JP, Hu Y, Pulmonary embolism and deep vein thrombosis caused by nitrous oxide abuse: A case report: World J Clin Case, 2019; 7; 4057-62
7.. Weimann J, Toxicity of nitrous oxide: Best Prac Res Clin Anaesthesiol, 2003; 17; 47-61
8.. McDermott R, Tsang K, Hamilton N, Belton M, Recreational nitrous oxide inhalation as a rare cause of spontaneous pneumomediastinum: Br Med J Case Rep, 2015; 2015; bcr2015209750
9.. Amess JA, Burman JF, Rees GM, Megaloblastic haemopoiesis in patients receiving nitrous oxide: Lancet (London, England), 1978; 2; 339-42
10.. Leslie K, Myles PS, Chan MT, Nitrous oxide and long-term morbidity and mortality in the ENIGMA trial: Anesth Analg, 2011; 112; 387-93
11.. Myles PS, Chan MT, Leslie K, Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery: Br J Anaesth, 2008; 100; 780-86
12.. Potocka-Banas B, Majdanik S, Dutkiewicz G, Death caused by addictive inhalation of nitrous oxide: Human Exp Toxicol, 2011; 30; 1875-77
13.. Richardson PG, Peripheral neuropathy following nitrous oxide abuse: Emerg Med Australas, 2010; 22; 88-90
14.. Hirvioja J, Joutsa J, Wahlsten P, Korpela J, Recurrent paraparesis and death of a patient with ‘whippet’ abuse: Oxford Med Case Rep, 2016; 2016; 41-43
15.. Egan W, Steinberg E, Rose J, Vitamin B(12) deficiency-induced neuropathy secondary to prolonged recreational use of nitrous oxide: Am J Emerg Med, 2018; 36; 1717 e1–.e2
16.. Schilling RF, Is nitrous oxide a dangerous anesthetic for vitamin B12-deficient subjects?: JAMA, 1986; 255; 1605-6
17.. Waclawik AJ, Luzzio CC, Juhasz-Pocsine K, Hamilton V, Myeloneuropathy from nitrous oxide abuse: Unusually high methylmalonic acid and homo-cysteine levels: Wis Med J, 2003; 102; 43-45
18.. Green R, Kinsella LJ, Current concepts in the diagnosis of cobalamin deficiency: Neurology, 1995; 45; 1435-40
19.. Harrington DJ, Laboratory assessment of vitamin B12 status: J Clin Pathol, 2017; 70; 168-73
20.. Wells PS, Anderson DR, Rodger M, Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: Increasing the models utility with the SimpliRED D-dimer: Thromb Hemost, 2000; 83; 416-20
21.. Caldera A, Mora J, Kotler M, Eiger G, Pulmonary embolism in a patient with pernicious anemia and hyperhomocysteinemia: Chest, 2002; 122; 1487-88
22.. Lupi-Herrera E, Soto-López ME, Lugo-Dimas AJ, Polymorphisms C677T and A1298C of MTHFR gene: Homocysteine levels and prothrombotic bio-markers in coronary and pulmonary thromboembolic disease: Clin Appl Thromb Hemost, 2019; 25; 1076029618780344
23.. Cellai AP, Lami D, Antonucci E, Hyperhomocysteinemia in patients with pulmonary embolism is associated with impaired plasma fibrinolytic capacity: J Thromb Thrombolysis, 2014; 38; 45-49
24.. Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M, Homocysteine-lowering in-terventions for preventing cardiovascular events: Cochrane Database Syst Rev, 2017; 8; CD006612
25.. Wald DS, Law M, Morris JK, Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis: BMJ (Clin Res ed), 2002; 325; 1202
26.. den Uil SH, Vermeulen EGJ, Metz R, Aortic arch thrombus caused by nitrous oxide abuse: J Vasc Surg Cases Innov Tech, 2018; 4; 80-82
27.. Liu M, Zhang J, Bu B, Isolated cortical vein thrombosis after nitrous oxide use in a young woman: A case report: BMC Neurol, 2020; 20; 378
Figures
In Press
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250