26 September 2021: Articles 
Pulmonary Tuberculosis After Therapy with Anti-Tumor Necrosis Factor (TNF) for Crohn Disease: A Case Report
Challenging differential diagnosis, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Douglas Inomata Cardoso da SilvaDOI: 10.12659/AJCR.932963
Am J Case Rep 2021; 22:e932963
Abstract
BACKGROUND: Adalimumab is a biological anti-tumor necrosis factor (TNF) agent which induces and maintains remission in patients with moderate-to-severe Crohn disease (CD). An adverse effect of its use is reactivation of latent infections, such as tuberculosis (TB). TB is caused by Mycobacterium tuberculosis and continues to be an important public health problem in some developing countries, such as Brazil. The present report describes the case of a patient with CD who developed pulmonary TB while receiving adalimumab therapy.
CASE REPORT: A 38-year-old penitentiary worker presented with colonic CD that was intolerant to azathioprine and was started on adalimumab. After 3 months, he experienced coughing, fever, and weight loss, and was diagnosed with pulmonary TB. A chest X-ray and tuberculin skin test performed before he started taking adalimumab were negative for latent TB. The patient was treated for 9 months to cure his infection. The use of adalimumab was suspended while the TB was investigated and he took mesalazine to achieve clinical and endoscopic remission of CD.
CONCLUSIONS: Adequate screening and chemoprophylaxis for latent TB are indicated in patients at high risk of infection. In patients with inflammatory bowel disease, after anti-TNF therapy is started, strict monitoring is required so that opportunistic infections can be detected early and morbidity and mortality reduced in this population.
Keywords: adalimumab, Crohn Disease, Inflammatory Bowel Diseases, latent tuberculosis, Humans, infliximab, Tuberculosis, Pulmonary, Tumor Necrosis Factor Inhibitors
Background
Crohn disease (CD), a chronic inflammatory condition that affects the gastrointestinal tract, results from interactions among genetic factors, environmental triggers, and unregulated immune responses [1]. The disease course is characterized by periods of relapse and remission at variable intervals, which greatly impact patient quality of life and functional capacity. The most frequent clinical manifestations are abdominal pain and diarrhea [1].
Adalimumab is a biological agent that is indicated for induction and maintenance of remission in patients with moderate-to-severe CD, and it is associated with control of the inflammatory process and healing of intestinal lesions [1]. Its mechanism of action is based on inhibition of tumor necrosis factor (TNF)-alpha, a pro-inflammatory cytokine involved in dysregulation of the immune response [1]. Despite the drug’s acceptable safety profile, a possible complication of use of adalimumab is reactivation of latent infections, such as tuberculosis (TB).
TB is caused by the bacterium
Patients treated with anti-TNF therapy such as adalimumab are at increased risk of developing opportunistic infections and reactivation of latent infections such as TB. This is not a new issue, but the present report describes a patient with CD who developed pulmonary TB after starting adalimumab therapy. Therefore, it underscores the importance of rigorous screening for this type of infection in patients with inflammatory bowel disease (IBD). Also included here is a discussion about the use of chemoprophylaxis with isoniazid in patients at high risk of TB infection.
The present study was approved by the local Research Ethics Committee (CAAE: 39827820.9.0000.5411). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Case Report
In February 2019, a 38-year-old man sought emergency treatment for diarrhea, bloody bowel movements, persistent abdominal pain, fever, and weight loss (<10% of body weight) that had occurred during the past few months. He lived in a rural city in the interior of the state of São Paulo, Brazil, and managed water and sewage treatment in a penitentiary unit. He reported no comorbidities and had no history of smoking.
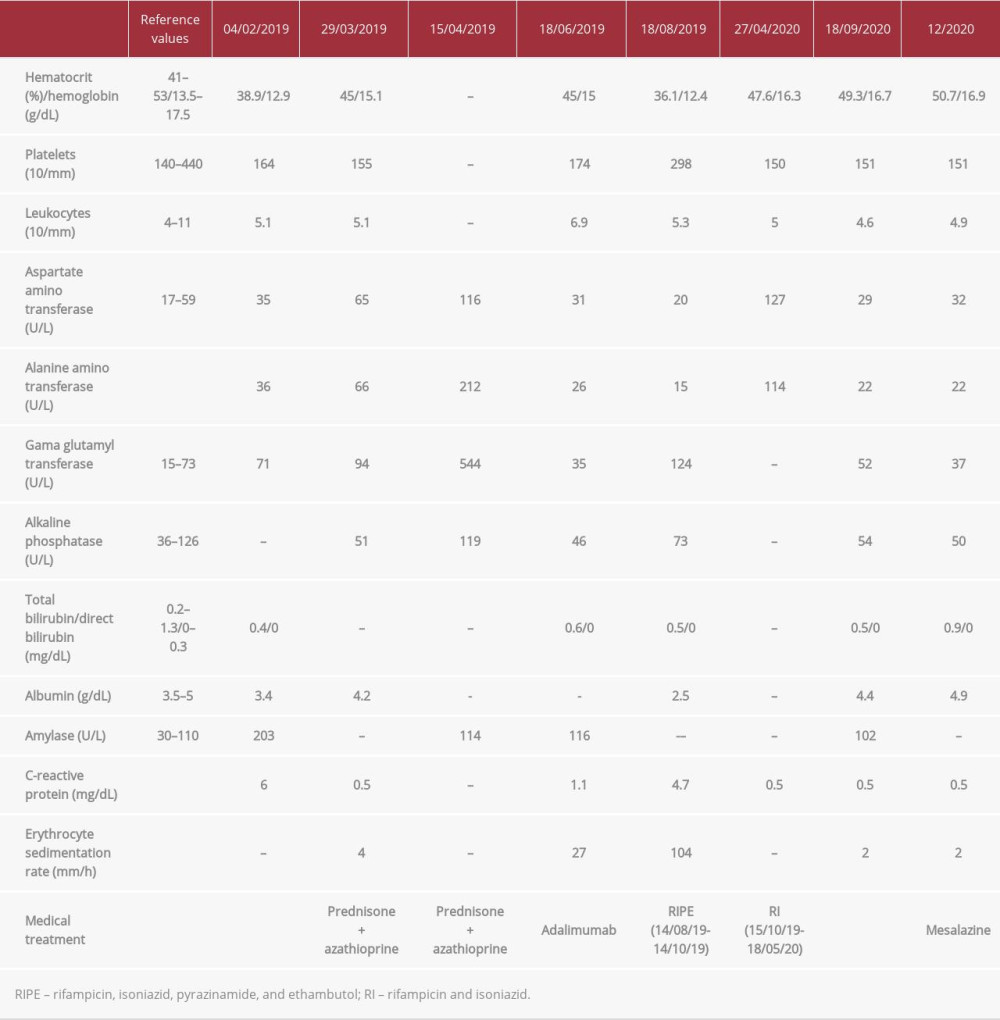
On physical examination, the patient’s abdomen was flat and he had pain on diffuse palpation, with no signs of peritonitis. Blood was observed on rectal examination. Biochemical tests revealed hemoglobin, 12.9 g/dL; hematocrit, 38.9%; and C-reactive protein, 6 mg/dL (normal range, <1.0 mg/dL) (Table 1). A colonoscopy showed terminal ileum with no injuries, presence of erosive lesions and longitudinal ulcers in the transverse and sigmoid colon, and friability in the rectum, consistent with active colonic CD. Histopathological examination showed moderate and intense colitis, presence of cryptitis, crypt micro-abscesses, and no granulomas. The patient underwent abdominal computed tomography (CT), which showed parietal thickening of the colon and no apparent lesions in the small intestine. Based on his clinical history and the results from colonoscopy and histopathology, he was diagnosed with CD.
The patient was started on treatment with prednisone (40 mg/d) and azathioprine 150 mg/d (2.3 mg/kg/d). After 30 days, his liver enzymes were elevated, suggestive of drug-induced hepatitis (Table 1), and the azathioprine was discontinued. We then opted to treat him with adalimumab. Before the drug was started, he had a 0-mm tuberculin skin test, a normal X-ray (Figure 1), and his serology was negative for HIV and hepatitis B and C. In June 2019, 3 months after treatment initiation, the patient presented to the Emergency Department with a persistent fever, dry cough, malaise, and hyporexia, with a weight loss of 8 kg in 3 weeks (>10% of body weight). Pulmonary auscultation revealed a vesicular murmur, which was globally reduced, and no other abnormalities. A chest X-ray showed consolidation of the pulmonary parenchyma, with alveolar and inter-stitial opacities in the anterior segment of the left upper lobe (Figure 2). A chest CT showed consolidation of the pulmonary parenchyma, bordered by ground-glass opacities and poorly defined alveolar nodules, with discrete air bronchograms in the medial portion of the anterior segment of the left upper lobe. Randomly distributed lung micronodules measuring up to 0.3 cm were seen in both lungs; enlarged lymph nodes also were found in the mediastinal region. These findings were compatible with an infectious process of fungal etiology; cryptococcosis and histoplasmosis were considered (Figure 3).
The patient underwent bronchoscopy for diagnostic clarification, which showed a mild inflammatory process in the left bronchial tree and a small, yellowish lesion in the lingual mucosa. Bronchoalveolar lavage was negative for acid-alcohol-resistant bacillus, gram-positive bacteria, and fungi, but positive for Koch’s bacillus. Treatment for pulmonary TB was initiated with an induction regimen consisting of 150 mg of rifampicin, 75 mg of isoniazid, 400 mg of pyrazinamide, and 275 mg of ethambutol hydrochloride (4 tablets/day for 2 months), followed by a maintenance schedule consisting of 300 mg of rifampicin and 200 mg of isoniazid (2 times/day for 9 months because of resistance to isoniazid despite clinical improvement). In May 2020, at the end of the regimen, the patient had recovered the weight he had lost and was asymptomatic.
Adalimumab was discontinued after the TB diagnosis was confirmed, because of its likely association with reactivation of latent infection. During treatment for TB, the patient experienced no symptoms of CD. One month after the end of the therapy, he experienced abdominal discomfort as his only symptom. A colonoscopy performed in August 2020 showed CD with mild activity in the distal segment of the rectum; rectal mesalazine (1 g/day) was prescribed. In November 2020, another colonoscopy was performed, which showed complete remission of the lesions (Figure 4).
Discussion
In recent decades, the incidence of IBD in Brazil has progressively increased. A recent systematic review that included studies from Latin America and the Caribbean revealed an increase in incidence in Brazil, from 0.68 per 100 000 people per year between 1991 and 1995 to 5.5 per 100 000 people per year in 2015 [4]. Over the years, more effective therapies have been developed, including TNF-alpha antagonists, which are immunobiological agents that have revolutionized the treatment of IBD and have the ability to modify the natural course of the disease and improve the disease prognosis and patient quality of life [5]. In Brazil, the increase in incidence of IBD was accompanied by an increase in the number of patients with CD using immunobiological agents, from 29.6% between 2005 and 2012 to 43.4% between 2013 and 2014 [4].
Despite their effectiveness in controlling symptoms, and inducing and maintaining remission of intestinal lesions, anti-TNF-alpha agents are associated with an increased risk of developing opportunistic infections such as TB [3,5]. In Brazil, TB is endemic and a serious and challenging public health problem. Although its incidence in Brazil trended downward between 2011 and 2016, it increased between 2017 and 2019. In 2020, however, notification about cases dropped because of the COVID-19 pandemic but a record 31.6 new cases per 100 000 inhabitants were recorded [6]. In 2019, 4500 deaths from the disease were reported, with a mortality rate of 2.2 deaths per 100 000 inhabitants [6]. Brazil is one of 30 countries with a high incidence of TB and is considered a priority for disease control by the World Health Organization (WHO). Worldwide, the WHO estimates that in 2019, about 10 million people developed TB and 1.4 million died from it [7].
The number of people infected with TB is believed to be even greater, because approximately one-quarter of the world’s population has the infection in its latent form, constituting bacillus reservoirs that have potential to be reactivated under conditions of altered immune response [8]. In individuals with latent TB, the use of anti-TNF-alpha agents such as adalimumab can further increase the risk of developing the disease, because they act by inhibiting the action of the pro-inflammatory cytokine TNF-alpha, which is involved in maintaining the integrity of the granuloma and is responsible for containing
This association between the use of anti-TNF-alpha and reactivation of latent TB was first identified when infliximab became available in 2001. Keane et al made the temporal connection based on findings in a series of patients who developed TB after being started on immunobiological agents [10]. To reduce this risk, screening for latent TB is required in all patients for whom use of a biological agent is anticipated. Standard screening includes a thorough medical history, with identification of epidemiological risk factors such as exposure to personal risk factors or family history of TB, as well as the presence of symptoms such as a dry or productive cough for 3 weeks or more, fever, weight loss, night sweats, chest pain, dyspnea, and asthenia; physical examination; and a chest X-ray. A tuberculin skin test also is required with the purified protein derivative (PPD), which has a mechanism of action based on late hypersensitivity to the purified protein derived from
In the present report, TB screening was performed with chest X-ray and PPD testing, both of which were negative. It is important to note that the tuberculin skin test was done while the patient was taking immunosuppressants (azathioprine and prednisone), which may have affected the results. There is great concern about the diagnostic validity of PPD in identifying patients who have a latent
A more recent alternative to PPD is the interferon-gamma release assay (IGRA) release test, based on identification of interferon-gamma, an inflammatory mediator released by T cells that have been stimulated by purified or synthesized
The results of comparative analyses of the sensitivity of the tests are controversial, and these studies have been conducted mainly in individuals who are immunosuppressed, which is the case in most patients with IBD. Use of immunosuppressants is known to increase the chance of false-negative PPD results [12,14]; therefore, some authors suggest that IGRA is a more sensitive test for detection of latent TB compared to PPD, and they prefer to use it for screening for that purpose [15,16]. Other studies, however, have shown a negative influence of immunosuppression on IGRA sensitivity, and as a result, the authors recommend that screening be performed before the start of immunosuppressive therapy. It is important to understand that comparing the accuracy of the tests is difficult because of the absence of a criterion standard for diagnosis of latent TB [17,18].
In view of the sensitivity and specificity limitations of PPD and the advent of IGRA and new studies in this area, international screening guidelines for TB have been revised and recommendations are based on the prevalence of TB and the resources available in each country or region. Therefore, in the United States, the current indication is to use IGRA rather than PPD when screening for latent TB infection in any patient [19]. In Brazil, the tuberculin skin test has been the standard test for screening for latent TB, and until recently it was the only test available in the public health system because of its low cost, ease of use, and the need for independent interpretation of more complex laboratory processes. However, following the global trend and given the limitations of PPD, IGRA testing recently has been incorporated into the public health system as an aid for diagnosis of latent TB in patients who are candidates for use of immunobiological agents and organ transplantation [20].
A recently published meta-analysis evaluated the factors associated with increased risk of TB in patients with IBD who are exposed to infliximab or adalimumab, with a special focus on local TB incidence [21]. Of the 130 114 patients studied, 373 developed TB and the risk was high in countries with a high incidence of tuberculosis (>40/100 000). The authors emphasized that 73% of patients who developed TB had no evidence of latent disease on screening, as was observed in our case. The authors discussed this issue and suggested that the patients probably developed reactivation rather than a primary infection, based on the short interval between the initiation of anti-TNF-alpha therapy and the development of TB [21]. A retrospective study performed in the northeast region of Brazil evaluated the risk of active TB in patients with IBD who were treated with anti-TNF-alpha monotherapy or in association with azathioprine [22]. The authors showed that the use of these 2 drugs increased the relative risk of active TB to more than 17.8 times that with conventional therapy [22].
It is important to note that the patient in the present case had an important occupational risk for development of TB because he managed sewage treatment in a penitentiary unit. However, this risk factor was not taken into account by the health team when treatment was started, and the medication was provided based on negative tests for latent TB. Policies for continuing medical education and awareness campaigns about the diagnosis of latent TB should be encouraged throughout the country, given the high prevalence of the disease, especially in vulnerable populations, such as those who have previously been proven to be TB-free. In view of the exposure to this important risk factor and the benefits of using anti-TNF-alpha, isoniazid chemoprophylaxis would have been indicated for the patient in the present case, starting at least 30 days before the introduction of adalimumab and maintained for 6 months. An alternative would have been the introduction of more target-specific biologicals, such as vedolizumab and ustekinumab, which have a lower risk of TB development [23,24], but these medications are not currently available for treatment of CD in the public health system in Brazil. Based on that, we opted to use mesalazine, despite its limitations in patients with CD.
Another point that deserves to be highlighted is the risk of developing immune reconstitution syndrome, which is characterized by paradoxical clinical and radiological worsening of TB after the introduction of anti-TB therapy. This syndrome is commonly seen in patients with HIV who are coinfected with TB after taking highly active antiretroviral therapy and experience recovery of their CD4 T-cell population, but it also has been described in patients with CD after discontinuation of anti-TNF-alpha therapy [25]. Although the patient in the present case did not experience this, it is important to mention the importance of detecting the syndrome in individuals who have an unsatisfactory response to treatment instituted for TB.
Conclusions
In a country such as Brazil in which TB is endemic, the use of biological anti-TNF-alpha agents needs to be considered carefully. It is essential to screen for latent TB, use chemoprophylaxis in high-risk patients, and strictly monitor patients after biological agents have been started so that any complications associated with the therapy can be identified early, diagnosed, and managed, thereby decreasing morbidity and mortality in this population.
Figures
References:
1.. Gomollón F, Dignass A, Annese V, 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and medical management: J Crohns Colitis, 2017; 11(1); 3-25
2.. , Tuberculosis control manual [Internet], 2019; 364 Available from: http://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf
3.. Keane J, TNF-blocking agents and tuberculosis: New drugs illuminate an old topic: Rheumatology (Oxford), 2005; 44(6); 714-20
4.. Kotze PG, Underwood FE, Damião AOMC, Progression of inflammatory bowel diseases throughout latin america and the caribbean: A systematic review: Clin Gastroenterol Hepatol, 2020; 18(2); 304-12
5.. Levin AD, Wildenberg ME, van den Brink GR, Mechanism of action of anti-TNF therapy in inflammatory bowel disease: J Crohns Colitis, 2016; 10(8); 989-97
6.. , 2021 Epidemiological Report – Tuberculosis 2021. ISSN 9352-7864
7.. , Global tuberculosis report 2020, 2020 Avaliable from: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf
8.. Cohen A, Mathiasen VD, Schön T, Wejse C, The global prevalence of latent tuberculosis: A systematic review and meta-analysis: Eur Respir J, 2019; 54(3); 1900655
9.. Gardam MA, Keystone EC, Menzies R, Anti-tumour necrosis factor agents and tuberculosis risk: Mechanisms of action and clinical management: Lancet Infect Dis, 2003; 3(3); 148-55
10.. Keane J, Gershon S, Wise RP, Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent: N Engl J Med, 2001; 345(15); 1098-104
11.. Jauregui-Amezaga A, Turon F, Ordás I, Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening: J Crohns Colitis, 2013; 7(3); 208-12
12.. Debeuckelaere C, De Munter P, Van Bleyenbergh P, Tuberculosis infection following anti-TNF therapy in inflammatory bowel disease, despite negative screening: J Crohns Colitis, 2014; 8(6); 550-57
13.. Abitbol Y, Laharie D, Cosnes J, negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: A descriptive study on the GETAID Cohort: J Crohns Colitis, 2016; 10(10); 1179-85
14.. Shim TS, Diagnosis and treatment of latent tuberculosis infection in patients with inflammatory bowel diseases due to initiation of anti-tumor necrosis factor therapy: Intest Res, 2014; 12(1); 12-19
15.. Schoepfer AM, Flogerzi B, Fallegger S, Comparison of interferon-gamma release assay versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease: Am J Gastroenterol, 2008; 103(11); 2799-806
16.. Richeldi L, Losi M, D’Amico R, Performance of tests for latent tuberculosis in different groups of immunocompromised patients: Chest, 2009; 136(1); 198-204
17.. Shahidi N, Fu YT, Qian H, Bressler B, Performance of interferon-gamma release assays in patients with inflammatory bowel disease: A systematic review and meta-analysis: Inflamm Bowel Dis, 2012; 18(11); 2034-42
18.. Wong SH, Gao Q, Tsoi KK, Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: A systematic review and meta-analysis: Thorax, 2016; 71(1); 64-72
19.. Mazurek GH, Jereb J, Vernon A: MMWR Recomm Rep, 2010; 59(RR-5); 1-25
20.. , Department of Science, Technology, Innovation and Strategic Health Inputs. Interferon-gamma release assay (IGRA) for detection of latent tuberculosis in immunocompromised patients, 2020 Available from: http://conitec.gov.br/images/Relatorios/2020/Relatorio_Recomendacao_InterfonGama_CP_47_2020.pdf
21.. Kedia S, Mouli VP, Kamat N, Risk of tuberculosis in patients with inflammatory bowel disease on infliximab or adalimumab is dependent on the local disease burden of tuberculosis: A systematic review and meta-analysis: Am J Gastroenterol, 2020; 115(3); 340-49
22.. Fortes FML, Sorte NB, Mariano VD, Active tuberculosis in inflammatory bowel disease patients under treatment from an endemic area in Latin America: World J Gastroenterol, 2020; 26(44); 6993-7004
23.. Souto A, Maneiro JR, Salgado E, Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: A systematic review and meta-analysis of randomized controlled trials and long-term extension studies: Rheumatology (Oxford), 2014; 53(10); 1872-85
24.. Ng SC, Hilmi IN, Blake A, Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting: Inflamm Bowel Dis, 2018; 24(11); 2431-41
25.. O’Dowd C, Kewin P, Morris J, Cotton M, Tuberculosis complicated by im-mune reconstitution inflammatory syndrome in a patient on anti-TNF- a therapy for Crohn’s disease: BMJ Case Rep, 2011; 2011; bcr0920103376
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250