10 November 2021: Articles 
Use of Argatroban in Donor Lung Procurement: A Case Report
Unusual setting of medical care
Limi Sharif1ABCDEF, M. Andrew Millis2ABCDE, Caitlin T. Demarest1AB, Ashraf Abou El Ela2AB, Katie A. McMurry3BEF, Dennis LyuDOI: 10.12659/AJCR.934054
Am J Case Rep 2021; 22:e934054
Abstract
BACKGROUND: Heparin-induced thrombocytopenia (HIT) is an immunological response to heparin exposure that predisposes patients to hypercoagulable reactions with subsequent heparin administration. Traditionally, heparin is the standard anticoagulant used during organ procurement to prevent clot formation in grafts. This creates a problem in donors or recipients that develop HIT as they are at risk of developing life-threatening coagulopathy. This raises the question of how to use alternative anticoagulation therapies, such as argatroban, that provide rapid-onset prophylaxis by reversibly inhibiting thrombin. Additionally, there are few studies that have assessed how recipients of multiorgan donors treated with argatroban do post-operatively.
CASE REPORT: In this report, we discuss the procurement protocol and hospital course of a lung transplant recipient who received a graft treated with argatroban due to a HIT-positive liver recipient. The post-operative course for our patient was uneventful, with improved lung function and no complications attributable to argatroban use. Further, none of the 4 other recipients who received organs from the same donor experienced graft dysfunctions secondary to coagulopathy, including the HIT-positive liver recipient.
CONCLUSIONS: The ultimate success of grafts without thromboembolic complications suggests the use of argatroban in multiorgan procurement in the setting of a HIT-positive recipient is safe and effective. This case report highlights an alternative to the traditional process of organ procurement with heparin, in which patients at risk of coagulopathies secondary to HIT are able to receive organs when traditional protocols would otherwise be prohibitive.
Keywords: Argatroban, Heparin, Lung Transplantation, Tissue and Organ Procurement, Anticoagulants, Arginine, Humans, Lung, Pipecolic Acids, Sulfonamides
Background
Patients with disorders of coagulation, such as heparin-induced thrombocytopenia (HIT), are a unique population in transplantation, as typical anticoagulation protocols use heparin to maintain organ viability during the procurement process [1]. HIT-positive patients manifest immune-mediated drug reactions associated with thrombocytopenia and life-threatening hypercoagulation [2]. Use of direct thrombin inhibitors such as argatroban provide similar anticoagulation benefits through an alternative mechanism, thereby allowing HIT-positive patients to receive organs while avoiding unwanted antibody activation [3]. While there is some literature on the use of these treatments in individual anticoagulation recipients, data on their use in multiorgan procurement are lacking [4,5]. Additionally, there are no standard protocols for anticoagulation in multiorgan procurement with HIT-positive recipients, and prior studies have involved flushing heparin, IVIG administration, and direct thrombin inhibitors [6–8].
The pathogenesis of HIT involves an immune-mediated response by IgG antibodies that attack platelet factor 4, a chemokine from activated platelets, creating a unit that attaches to platelets, causing further aggregation. Thrombin is released with widespread platelet aggregation, putting patients in a hypercoagulable state [2,9]. Mortality is estimated to be around 5–10%, especially with delayed diagnosis, with an incidence in surgical patients of up to 5% [10].
Anticoagulation therapies using direct thrombin inhibitors such as argatroban work by reversibly binding to the active thrombin site of free and clot-associated thrombin for rapid-onset, short-duration HIT prophylaxis and treatment [3]. The in vivo half-life of argatroban is 39–51 min and reaches steady state in 1–3 h in patients with normal liver function [11]. When compared to heparin, these agents are more efficacious across a broader range of populations given that they bind directly to thrombin and have more predictable pharmacokinetics [12,13]. In patients with active HIT, argatroban is shown to decrease the rate of death, stroke, amputation, and new thromboses [14,15]. However, the safety of argatroban for organ procurement for transplantation has not been extensively evaluated.
In this case report, we describe the hospital course of a lung recipient of a multiorgan donor treated with argatroban due to a HIT-positive liver recipient. Our report is unique in that it discusses argatroban use in the multiorgan procurement phase and describes continued graft function in the lung transplant recipient. Further, in spite of an argatroban-treated donor, the 4 other graft recipients continued to maintain graft function following transplantation, including the HIT-positive liver recipient. Our experience adds to the limited number of case studies that demonstrate the safe and effective use of argatroban in the organ procurement phase, particularly in lung transplant recipients.
Case Report
RECIPIENT INFORMATION:
Our recipient was a 57-year-old woman with alfa-1 antitrypsin deficiency and severe emphysema. Pertinent preoperative lab results included an international normalized ratio (INR): 1.1, partial thromboplastin time (PTT): 25.8, forced vital capacity (FVC): 1.88 (70% predicted), forced expiratory volume (FEV1): 0.54 (27% predicted), FEV1/FVC ratio: 28.72 (39% predicted). She did not have a history significant for HIT.
DONOR INFORMATION:
The donor was a 22-year-old woman with a cause of death due to complications from anoxic brain injury secondary to drug overdose. Arterial blood gas analysis showed a partial tension of carbon dioxide of 27.7–35.0 and a mean partial oxygen tension of 147.1–454.9 on 100% oxygen challenge. Imaging with chest X-ray and CT scan showed areas of ground-glass opacities bilaterally and increased density in both lung bases. Her lungs appeared normal during onsite evaluation with bronchoscopy.
PROTOCOL FOR ANTICOAGULATION:
The donor operation took place 4 days following declaration of brain death. She was initially anticoagulated with 5000 units of prophylactic heparin q12 h preoperatively, but this was stopped on the day of the donor operation once the liver was transplanted to a HIT-positive recipient. She then received 350 mcg/kg for a total of 26 mg of argatroban intra-operatively. Anti-IIa levels were then monitored every 4–6 h. Argatroban was administered 15 min prior to donor aortic cross-clamp with a target anti-IIa level of 0.4–1.2 mcg.
A standard multiorgan procurement proceeded with the heart, lungs, liver, and kidneys. The lungs were flushed with 4 L of Perfadex antegrade, 1 L Perfadex retrograde static flush, preserved, inflated to 15–20 cmH2O pressure, and put on ice at 4°C per standard protocols. We added 2000 units of heparin to the backflush due to concerns regarding thrombosis. They were transported to the University of Michigan hospital for transplant. The lungs were transported with a total time of 8 h from procurement to transplant.
IMPLANTATION:
The native left lung was removed, and transplant proceeded on the left side. We adapted our standard back bench flush protocol to include an additional heparin dose of 1000 units, as well as a second flush with Perfadex to prevent clot formation. Bronchial anastomoses were performed, and lungs were reperfused over a period of 15 min. The right donor lung was similarly prepared, and several small clots were flushed out during backflushing. The lung was reperfused over a period of 12 min after the anastomoses were complete. There were no intraoperative complications, and the patient received 5.5 L of crystalloid and 2 units of red blood cells.
POST-OPERATIVE COURSE:
The patient was extubated to room air on post-operative day (POD) 1. Antibiotic prophylaxis was started for donor cultures positive for
Notably, the patient had no thromboembolic or coagulopathic complications in the perioperative period. Immediate postoperative mean pulmonary artery pressure was 17, briefly reaching a maximum of 24 and a minimum of 11 before the Swan-Ganz catheter was removed on POD 2. She was placed on subcutaneous heparin 5000 U TID for venous thromboembolism prophylaxis. Of note, post-operative anti-IIa levels demonstrated an argatroban level of 0 following transplant. The patient’s coagulation profile (Table 1) remained within normal limits except on the day of transplant, which is reflective of anticoagulation during surgery. Additionally, reagent-modified rotational thromboelastometry (ROTEM; Table 2) values during this time period remained within normal limits, suggesting that the patient was not excessively anticoagulated. The patient’s pulmonary function tests showed marked improvement following transplant (Figure 1).
Discussion
The safety of argatroban for organ procurement for transplantation has not been extensively evaluate for efficacy or safety. Herein, we report a case in which argatroban appears to have had little impact on the ultimate successful donor preservation and lung transplantation. Throughout the perioperative period, the patient was neither under- nor over-anticoagulated. The INR, PT, and PTT were elevated immediately post-operatively but returned to baseline normal by POD 1, without reversal or product administration.
In our case, clotting time was within normal range and it is likely that minimal levels of argatroban, if any, were left in the graft at the time of transplantation, which is consistent with the fact that approximately 10 half-lives occurred between the time of argatroban administration and implantation, as well as the additional Perfadex/heparin flushes on the back-table. Argatroban use in this setting did not affect the recipient’s ability to form clots based on biochemical and coagulation studies, nor did it lead to greater than expected blood loss at the time of surgery.
There is only 1 other case report on the use of argatroban in multiorgan procurement, and it discusses the transfer of coagulopathy to a graft recipient in the setting of excess donor anticoagulation [8]. However, this does not preclude argatroban use, but rather enforces the need for careful dosing. Argatroban is initiated at 1 mcg/kg/min in patients without hepatic impairment and adjusted to 0.5 mcg/kg/min in patients with hepatic impairment per our hospital protocol. When used in appropriate doses, argatroban can be used in multiorgan procurement while avoiding unwanted immunological reactions in HIT-positive recipients.
The effect of argatroban might be more profound if argatroban were required in the preparatory phase in addition to the procurement phase. The use of argatroban in back-table flush solutions could leave an organ with a higher concentration of the drug than only systemic administration, as in our case. However, the United Network for Organ Sharing (UNOS) did note that there were no graft dysfunctions reported in the post-operative period for any of the other organ recipients, including the HIT-positive liver recipient. Hence, it is unlikely that the additional backflush we used affected the safety, but further data will need to be gathered to ensure that this is indeed safe.
Conclusions
Our patient did well post-operatively with few complications and no clearly identifiable complications due to argatroban. While heparin will likely remain the standard of care for anticoagulation in transplantation, the ultimate success of the grafts suggests the use of argatroban in multiorgan procurement in the setting of a HIT-positive recipient is safe and effective. In recipients who are HIT-positive, safe and effective use of argatroban enables them to receive organs when traditional protocols would otherwise prohibit this.
References:
1.. Korte C, Garber JL, Descourouez JL, Pharmacists’ guide to the management of organ donors after brain death: Am J Health System Pharm, 2016; 73(22); 1829-39
2.. Prince M, Wenham T, Heparin-induced thrombocytopaenia: Postgrad Med J, 2018; 94(1114); 453-57
3.. Mckeage K, Plosker GL, Argatroban: Drugs, 2001; 61(4); 515-22
4.. Koster A, Niedermeyer J, Gummert J, Renner A, Low dose bivalirudin anticoagulation for lung transplantation with extracorporeal membrane oxygenation in a patient with acute heparin-induced thrombocytopenia: Eur J Cardiothorac Surg, 2017; 51(5); 1009-11
5.. Lee SK, Cho WH, Kim DH, Yeo HJ, Lung transplantation using argatroban in severe heparin-induced thrombocytopenia during extracorporeal membrane oxygenation: A case series: Gen Thorac Cardiovasc Surg, 2020; 68(12); 1565-68
6.. Warkentin TE, High-dose intravenous immunoglobulin for the treatment and prevention of heparin-induced thrombocytopenia: A review: Expert Rev Hematol, 2019; 12(8); 685-98
7.. Bachmann R, Nadalin S, Li J, Donor heparinization is not a contraindication to liver transplantation even in recipients with acute heparin-induced thrombocytopenia type II: A case report and review of the literature: Transpl Int, 2011; 24(10); 89-92
8.. Schwartz JJ, Hatch JM, Book Z, Use of Argatroban during multi-organ procurement: Pharmacokinetics and sequelae in recipient of transplanted liver: Clin Transplant, 2009; 23(5); 705-9
9.. Greinacher A, Potzsch B, Amiral J, Heparin-associated thrombocytopenia: Isolation of the antibody and characterization of a multimolecular PF4-heparin complex as the major antigen: Thromb Hemost, 1994; 71(2); 247-51
10.. Battistelli S, Genovese A, Gori T, Heparin-induced thrombocytopenia in surgical patients: Am J Surg, 2010; 199(1); 43-51
11.. Koster A, Faraoni D, Levy JH, Argatroban and Bivalirudin for perioperative anticoagulation in cardiac surgery: Anesthesiology, 2018; 128(2); 390-400
12.. Fisser C, Winkler M, Malfertheiner M, Argatroban versus heparin in patients without heparin-induced thrombocytopenia during venovenous extracorporeal membrane oxygenation: A propensity-score matched study: Crit Care, 2021; 25(1); 1-10
13.. Coughlin MA, Bartlett RH, Anticoagulation for extracorporeal life support: Direct thrombin inhibitors and heparin: ASAIO J, 2015; 61(6); 652-55
14.. Yeh RW, Jang I, Argatroban: update: Am Heart J, 2006; 151(6); 1131-38
15.. Lamonte MP, Brown PM, Hursting MJ, Stroke in patients with heparin-induced thrombocytopenia and the effect of argatroban therapy: Crit Care Med, 2004; 32(4); 976-80
Tables
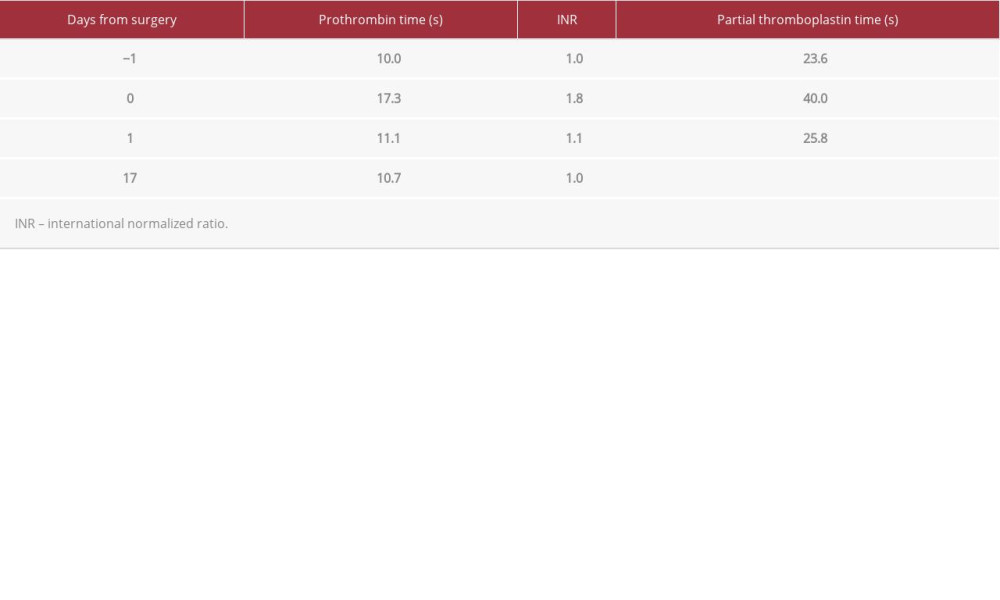 Table 1.. Coagulation profile of lung recipient pre- and post-operatively.
Table 1.. Coagulation profile of lung recipient pre- and post-operatively.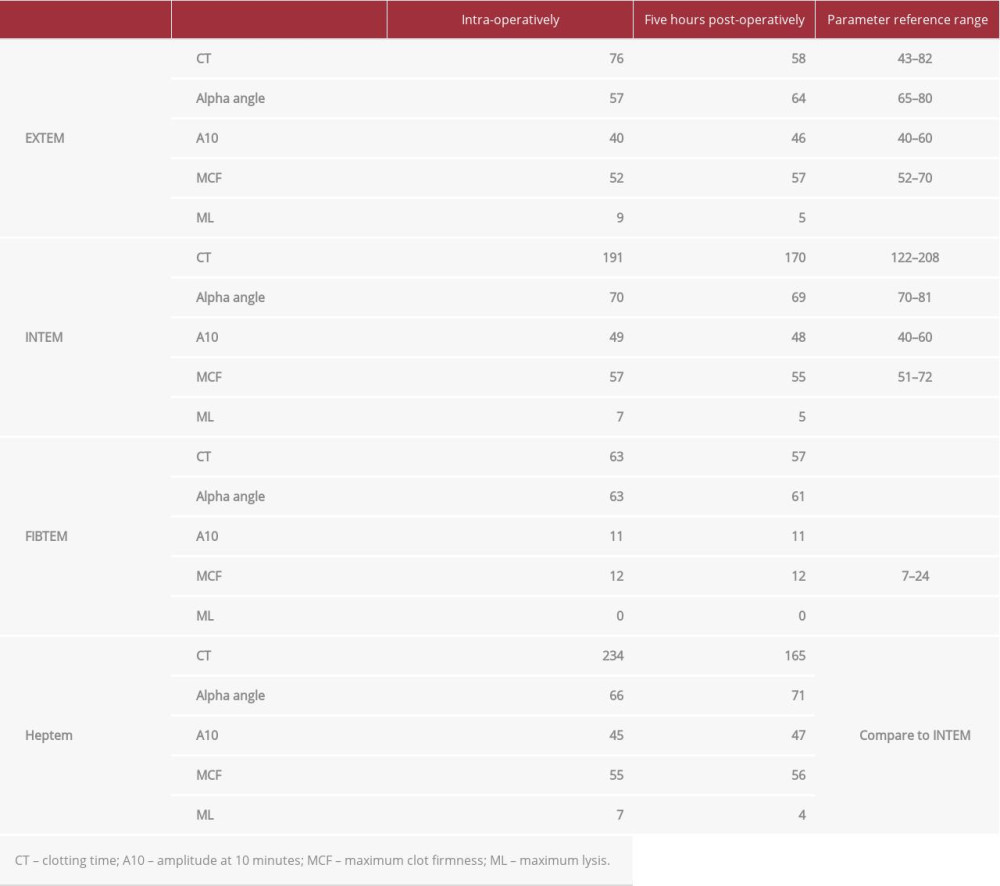 Table 2.. ROTEM values for lung recipient intra-operatively and immediately post-operatively.
Table 2.. ROTEM values for lung recipient intra-operatively and immediately post-operatively. Table 1.. Coagulation profile of lung recipient pre- and post-operatively.
Table 1.. Coagulation profile of lung recipient pre- and post-operatively. Table 2.. ROTEM values for lung recipient intra-operatively and immediately post-operatively.
Table 2.. ROTEM values for lung recipient intra-operatively and immediately post-operatively. In Press
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943687
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








