09 February 2022: Articles 
Extensive Cerebral Venous Sinus Thrombosis (CVST) After the First Dose of Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine without Thrombotic Thrombocytopenia Syndrome (TTS) in a Healthy Woman
Unusual clinical course, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Ali AlhashimDOI: 10.12659/AJCR.934744
Am J Case Rep 2022; 23:e934744
Abstract
BACKGROUND: COVID-19 is an acute respiratory disease caused by the SARS-CoV-2 virus, which was discovered in 2019. The high transmission and seriousness of COVID-19 necessitated the development of an effective vaccine to control spread of the disease. Multiple vaccines have been granted emergency use authorization (EUA) by the U.S. Food and Drug Administration, namely, the Pfizer-BioNTech, Moderna (mRNA), and the Johnson & Johnson/Janssen (vector) vaccines. As these novel vaccines have been used, adverse effects have been reported, ranging from mild myalgia to severe anaphylaxis and thrombotic events. Thrombotic consequences raised suspicion for the development of cerebral venous sinus thrombosis (CVST), which is a severe condition associated with occlusion of venous sinuses and disruption of the venous system flow.
CASE REPORT: A 28-year-old healthy woman presented with a 2-week history of persistent and progressive headache 4 days after receiving an mRNA COVID-19 vaccine (Pfizer-BioNTech). Cerebral computed tomography (CT) and CT venography confirmed the presence of extensive thrombus involving the left transverse and sigmoid sinus as well as the internal jugular vein. Furthermore, other than recent the COVID-19 vaccination, there were no precipitant risk factors in her clinical history or in the detailed laboratory work-up.
CONCLUSIONS: Headache associated with red flags following administration of any COVID-19 vaccine should prompt urgent neuroimaging to rule out secondary causes and determine the appropriate management. Our patient lacked the typical profile of CVST commonly seen following administration of the Oxford-Astrazeneca vaccine. The findings of low platelet count may indicate the peculiar pathophysiology of a thrombotic event associated with with the Pfizer vaccine.
Keywords: severe acute respiratory syndrome coronavirus 2, COVID-19 vaccine, Stroke, Adult, BNT162 Vaccine, COVID-19, COVID-19 Vaccines, Female, Humans, RNA, Messenger, SARS-CoV-2, Sinus Thrombosis, Intracranial, Thrombocytopenia, Thrombosis
Background
The world was struck by the emergence of coronavirus disease COVID-19, a severe respiratory syndrome caused by the SARSCoV-2 virus, a pandemic that we are still battling to this day. COVID-19 was identified in 2019 and great efforts have been made to establish safe and effective vaccines [1].
Three vaccines have been granted emergency use authorization (EUA) by the U.S. FDA: the Pfizer-BioNTech, Moderna (mRNA), and Johnson & Johnson/Janssen (vector) vaccines [2–4]. Each vaccine has a different mechanism of action. The mRNA-based vaccine uses RNA nanoparticles that incorporate into the cell cytoplasm, the vector-based vaccine is a genetically designed virus with modified DNA, and the protein subunits vaccine is a virus with engineered DNA fragments. All these vaccines are designed to produce coronavirus spike proteins and trigger an immune response [5].
As the novel vaccines began to be used, adverse effects were reported, ranging from mild arthralgia and myalgia to severe anaphylaxis and cerebral venous sinus thrombosis [2–12]. The incidence rate of CVST within the first 31 days following the first dose of COVID-19 vaccine in Germany was 0.55 per 100 000 individuals [13].
CVST is a rare, serious neurovascular condition defined as occlusion of venous sinuses that leads to a cascade of events. It increases the venous pressure, disrupts its return, and cause infarction and hemorrhage, which can eventually lead to poor outcomes [14]. Thrombosis of the dural sinus and/or cerebral veins (CVT) is an uncommon form of stroke, usually affecting young females [15,16]. The risk factors for venous thrombosis are classically linked to the Virchow triad, which includes stasis of blood, changes in the vessel wall, and changes in the composition of blood. Risk factors are usually divided into acquired risk factors (eg, surgery, trauma, pregnancy, puerperium, antiphospholipid syndrome, cancer, exogenous hormones, infection, and after vaccination) and genetic risk factors (eg, inherited thrombophilia) [14]. Due to the seriousness of CVST, studies have been paying special attention to the incidence of CVST after vaccination. Herein, we report a case of extensive CVST following administration of the first doses of the BNT162b2 (Pfizer-BioNTech) vaccine in a healthy woman.
Case Report
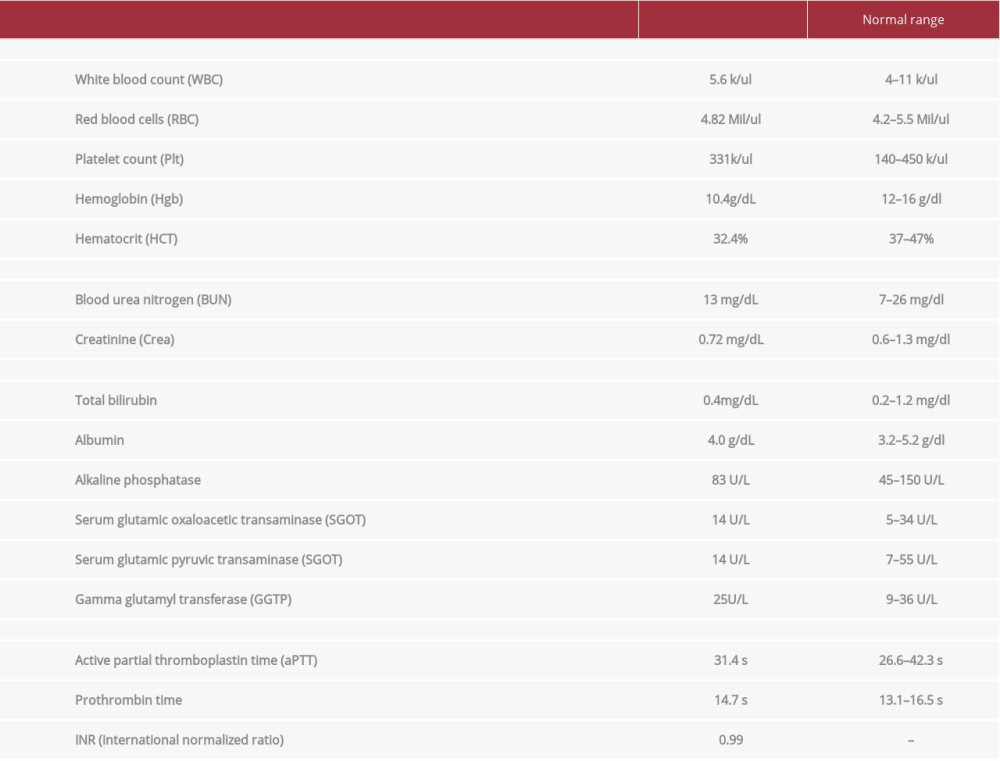
A 28-year-old, right-handed, healthy woman from Kenya presented to our neurology clinic with a 2-week history of persistent, progressive, left-sided headache. She described a moderate-to-severe (visual analog scale: 7–8/10) dull aching pain in the left temporal and occipital areas. It was associated with mild photophobia. The patient did not report having phonophobia, nausea, vomiting, or other neurological symptoms. There were no obvious aggravating or relieving factors. Furthermore, our patient mentioned previous transient recurrent attacks of mild-to-moderate tension-type headache since 2018. These attacks responded well to simple analgesics and lasted only for a few hours. However, her current presentation was different in location, pattern, severity, duration, and responsiveness to analgesia from that of past years. She had recently had multiple ER visits for intravenous analgesia due to inadequate response to over-the-counter analgesia. Systemic review and B-symptoms were negative. She reported no recent infection, especially no recent SARS-CoV-2 infection. Both past medical and surgical history were unremarkable for her condition except for the previously mentioned headache. Regarding drug history, she received the Pfizer-BioNTech mRNA vaccine 4 days prior to presentation and reported no use of oral contraceptive pills or any other medications of clinical relevance. Her family history was unremarkable for any relevant medical issues. She was single and had never been pregnant, and she had been working in Saudi Arabia for 8 months. She denied use of alcohol, tobacco, and recreational drugs. In physical examinationshe was vitally stable, afebrile, and normotensive with blood pressure of 117/76 mmHg and pulse 73 beats per minute. When a neurological examination was preformed, no focal neurological deficits were detected. Routine lab results (Table 1) were insignificant and her platelet count was normal.
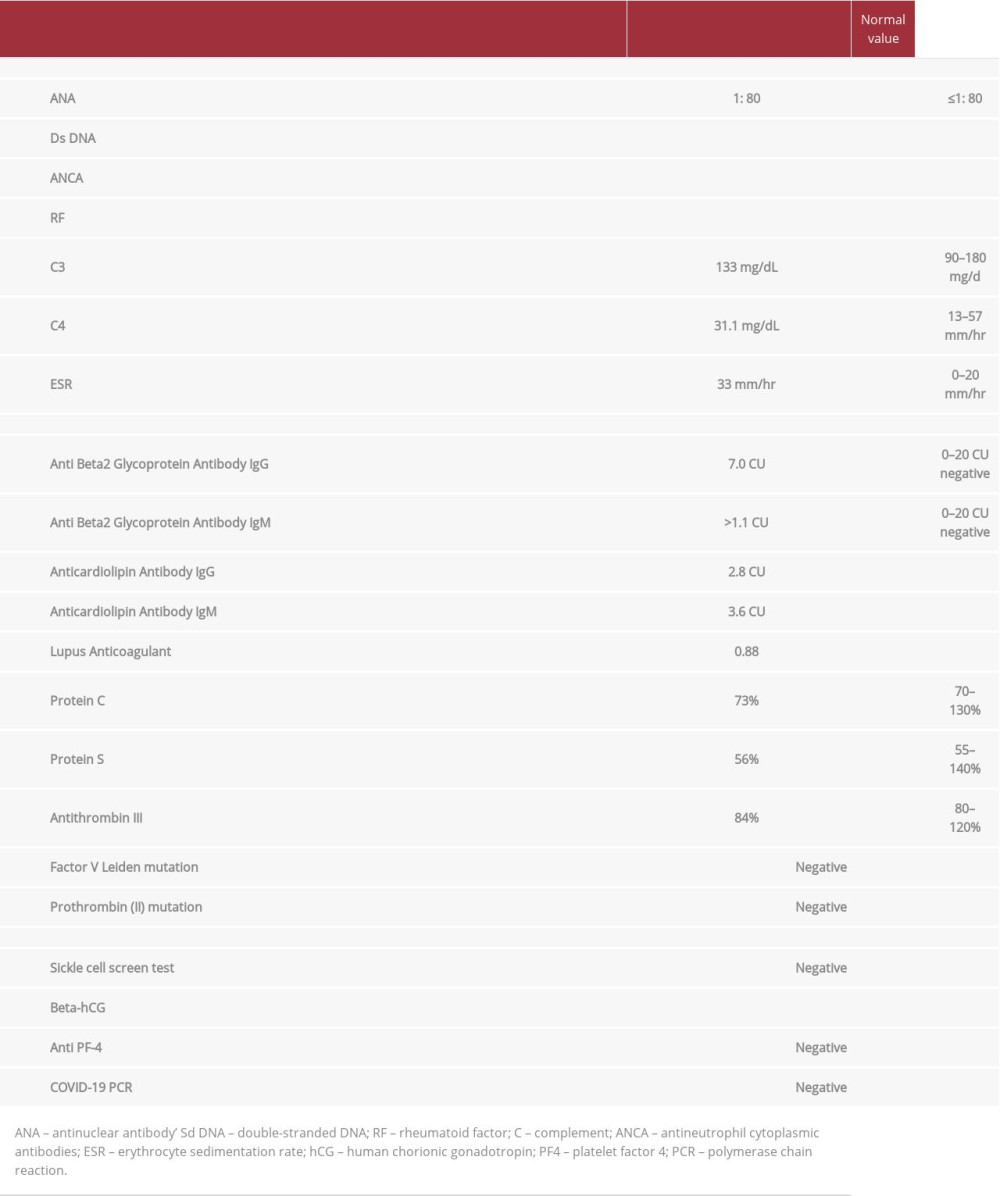
Because our patient’s clinical presentation, the persistence and progression of headache, as well as the lack of responsiveness to analgesia warranted further evaluation, we performed a cerebral computed tomography (CT) scan (Figure 1A). The CT scan showed a hyperdense sinus sign in the left transverse sinus, suggestive of a sinus thrombus; therefore, a complementary CT venography (Figures 1B–1D, and 2A, 2B) was done, revealed a filling defect within the confluent sinus extending through the left transverse sinus and sigmoid sinus up to the proximal segment of the internal jugular vein. All these CT findings were consistent with extensive cerebral venous sinus thrombosis (CVST). Since no clear precipitating factors were found in her clinical history, we ordered vasculitis (ANA, Ds DNS, RF, CRP, C3, C4) and thrombophilia (Protein C, S, Antithrombin III, Factor V and II mutation, anti B2GP1-IgG/IgM, anticardiolipin, lupus anticoagulant) work-up (Table 2). We also ordered anti-platelet factor 4 antibody (PF-4), sickle cell screen test, nasopharyngeal COVID-19 PCR, and a pregnancy test. The results of all these tests were negative.
We treated her with enoxaparin (65 mg bid) for 7 days, then she was switched to rivaroxaban, an oral direct factor Xa inhibitor. Her headache greatly improved and by day 3 it completely subsided without need for analgesics. The patient was discharged home in symptom-free status and a follow-up CTV was scheduled in 3 months.
Discussion
Headache is a common neurological manifestation encountered on a daily basis in emergency rooms and outpatient clinics. A systematic approach focusing on red flags is paramount to guide further laboratory and imaging to look for secondary headache. A “SNNOOP10” red flags list is a reasonable tool to guide urgent investigation of headache patients [9,17]. We stress this point because our patient had multiple ER visits and, despite the presence of red flag symptoms, no appropriate work-up had been done before she presented to our clinic. Importantly, most secondary headache can be life-threatening if not treated urgently [15–17].
CVST in most instances is triggered by single or multiple pre-disposing factors, even transient factors such as a systemic or local (ENT; ear nose throat) infection. Clinically, at least 1 precipitant risk factor was identified in more than 85% of cases, and multiple risk factors were found in about half [11]. In our patient, despite the comprehensive clinical approach (precise history and detailed laboratory work-up), no precipitant factors were identified except for a recent COVID-19 vaccination.
CVST is a rare adverse event observed recently with increasing numbers of COVID-19 vaccinations worldwide. Most COVID-19 vaccine-related CVST cases were associated with vector-based vaccine (ChAdOx1; Vaxzevria; previously AstraZeneca and Janssen/Johnson & Johnson). They are usually accompanied by thrombotic thrombocytopenic syndrome (TTS) [7,12,19,20]. However, our patient developed CVST without TTS following her first dose of mRNA COVID-19 vaccine (BENT126b2; Pofizer-biontech). So far, there are a scarce reports of mRNA COVID-19 vaccine-related CVST cases without TSS V in the literature [18,20,21].
The estimated incidence of CVST in the general population 1.31 to 2.78 per 100 000 [16,17]. The reporting event rate of CVST after BENT126b2 mRNA (Pfizer-bioNtech) from 12 December 2020 to 16 March 2021 was 0.4% (4 cases/1197), that was less than viral vector vaccine-related CVST within the given period, which was 1.1% (7 cases out of 639) [22]. Likewise, an analysis of CVST after vaccination in European countries noted that CVST after ChAdOx1 COVID-19 vaccination is by far more frequent than with mRNA vaccines [12]. Nevertheless, SARS-CoV-2 infection itself is associated with a markedly increased incidence of CVST compared to the general population, patients with influenza, and people who have received BNT162b2 or mRNA-1273 vaccines [23]. These data provide clinicians and patients reassurance that COVID-19 vaccines are safe and their benefits outweight the risk of SARS-CoV-2 infection [24].
The mechanism of CVST associated with viral vector vaccine is mainly vaccine-induced immune thrombotic thrombocytopenia (VITT), which is analogous to autoimmune heparin-induce thrombocytopenia (HIT) [11]. The mechanisms mRNA vaccine-related CVST is still unclear, but may be explained by the following. Firstly, the spike glycoprotein interaction with platelet leads to platelet aggregation [25]. Secondly, binding of spike glycoprotein to the angiotensin converting enzyme receptor cause activation of endothelial cells and upregulation of cell adhesion molecules (ICAM-1 and VCAM-1), this cascade can promote thrombogenesis and causes CVST [26]. This is quite similar to the thrombosis pathogenesis associated with COVID-19 infection [27]. Thirdly, in vitro studies have shown that spike proteins can activate an alternative pathway of complement system, which have a role in immune-mediated thrombogenesis [28].
Due to rarity of thrombotic events following administration of the mRNA COVID-19 vaccine, causality cannot be confirmed to date due to lack of statistical power. This case adds to the growing body of evidence that suggests the possible relationship between mRNA COVID-19 vaccination and thrombotic events, and raises the awareness of health care providers and the public about this rare critical adverse event, leading to early recognition and prevention of its compilations.
Conclusions
Headache with red flags such as a progressive and persistent headache following any COVID-19 vaccination should prompt urgent neuroimaging to rule out secondary causes and treat them accordingly. Our patient lacked the typical profile of cerebral venous thrombosis that is usually seen following administration of the Oxford-Astrazeneca vaccine such as low platelet counts, which may indicate peculiar pathophysiology of thrombotic event with the Pfizer vaccine. Large registeries are needed to establish if thrombotic events are merely incidental events or are an mRNA-based vaccine complication.
Figures
References:
1.. , Archived: WHO Timeline – COVID-19WHO [organization online] Apr, 2020; 1(1) [Citeded 2021 Sep 05]
2.. , FDA Approves First COVID-19 Vaccine. FDA [organization online] Aug, 2021; 1(1) [Citeded 2021 Sep 5];
3.. , Moderna COVID-19 Vaccine. FDA [organization online] Aug, 2021; 1(1) [Citeded 2021 Sep 5]
4.. , Janssen COVID-19 Vaccine. FDA [organization online] Aug, 2021; 1(1) [Citeded 2021 Sep 5]
5.. Mascellino MT, Di Timoteo F, De Angelis M, Oliva A, Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety: Infect Drug Resist, 2021; 14; 3459-76
6.. , Global Advisory Committee on Vaccine Safety (GACVS) review of latest evidence of rare adverse blood coagulation events with AstraZeneca COVID-19 Vaccine (Vaxzevria and Covishield). WHO [organization online] Apr, 2020 [Cited 2021 Sep 05]; 1(1):[3 screens]. Available from: https://www.who.int/news/item/1604-2021-global-advisory-committee-on-vaccine-safety-(gacvs)-review-of-latest-evidence-of-rare-adverse-blood-coagulation-events-with-astrazeneca-covid-19-vaccine-(vaxzevria-and-covishield)
7.. Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; A systematic review: J Neurol Sci, 2021; 428; 117607
8.. Perry RJ, Tamborska A, Singh B, CVT After Immunisation Against COVID-19 (CAIAC) collaborators. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: A multicentre cohort study: Lancet, 2021; 398; 1147-56
9.. Do TP, Remmers A, Schytz HW, Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list: Neurology, 2019; 92(3); 134-44
10.. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT): Stroke, 2004; 35(3); 664-70
11.. Rizk JG, Gupta A, Sardar P, Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: A review: JAMA Cardiol, 2021; 6; 1451-60
12.. Krzywicka K, Heldner MR, Sánchez van Kammen M, Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: An analysis of cases notified to the European Medicines Agency: Eur J Neurol, 2021; 28; 3656-62
13.. Schulz JB, Berlit P, Diener HC, German Society of Neurology SARSCoV-2 Vaccination Study Group. COVID-19 vaccine-associated cerebral venous thrombosis in Germany: Ann Neurol, 2021; 90(4); 627-39
14.. Saposnik G, Barinagarrementeria F, Brown RD, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the American Heart Association/American Stroke Association: Stroke, 2011; 42(4); 1158-92
15.. Coutinho JM, Ferro JM, Canhão P, Cerebral venous and sinus thrombosis in women: Stroke, 2009; 40(7); 2356-61
16.. Coutinho JM, Gerritsma JJ, Zuurbier SM, Stam J, Isolated cortical vein thrombosis: Systematic review of case reports and case series: Stroke, 2014; 45(6); 1836-38
17.. Ramanayake RPJC, Basnayake BMTK, Evaluation of red flags minimizes missing serious diseases in primary care: J Family Med Prim Care, 2018; 7(2); 315-18
18.. Fan BE, Shen JY, Lim XR, Cerebral venous thrombosis post BNT162b2 mRNA SARS-CoV-2 vaccination: A black swan event: Am J Hematol, 2021; 96(9); E357-61
19.. See I, Su JR, Lale A, US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021: JAMA, 2021; 325(24); 2448-56
20.. Syed K, Chaudhary H, Donato A, Central venous sinus thrombosis with subarachnoid hemorrhage following an mRNA COVID-19 vaccination: Are these reports merely co-incidental?: Am J Case Rep, 2021; 22; e933397
21.. Dias L, Soares-Dos-Reis R, Meira J, Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine: J Stroke Cerebrovasc Dis, 2021; 30(8); 105906
22.. Smadja DM, Yue QY, Chocron R, Vaccination against COVID-19: Insight from arterial and venous thrombosis occurrence using data from VigiBase: Eur Respir J, 2021; 58(1); 2100956
23.. Taquet M HM, Geddes JR, Luciano S, Harrison PJ, Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine: Stroke J, 2021 [Preprint Verison 2 posted online April 15, 2021]
24.. , COVID-19 Vaccine AstraZeneca: Benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. EMA [online] Mar, 2021 [Citeded 2021 Sep 5]; 1(1): [4 screens]. Available from: https://www.ema.europa.eu/en/news/covid-19-vaccine
25.. Zhang S, Liu Y, Wang X, SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19: J Hematol Oncol, 2020; 13(1); 120
26.. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier: Neurobiol Dis, 2020; 146; 105131
27.. Divani AA, Andalib S, Di Napoli M, Coronavirus disease 2019 and stroke: Clinical manifestations and pathophysiological insights: J Stroke Cerebrovasc Dis, 2020; 29(8); 104941
28.. Yu J, Yuan X, Chen H, Chaturvedi S, Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition: Blood, 2020; 136(18); 2080-89
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942966
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942032
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250