24 May 2022: Articles 
A 62-Year-Old Man Presenting with Bilateral Tremor of the Upper Limb After a Diagnosis of COVID-19 with Confirmed Volumetric Brain Loss
Unusual clinical course, Challenging differential diagnosis
Sachin M. Bhagavan1CDEF*, Swathi Beladakere Ramaswamy1DEF, Tejas R. Mehta1EF, Raghav Govindarajan1ADEF, Joseph Cousins2CDEFDOI: 10.12659/AJCR.934955
Am J Case Rep 2022; 23:e934955
Abstract
BACKGROUND: The SARS-CoV-2 viral infection is associated with respiratory and multi-organ systemic disease. It has been shown to affect the central nervous system and produce varied neurological symptoms, including ischemic strokes, seizures, and encephalitis. Neurological manifestations of this viral infection are thought to be due to neurotropic reactions on the central nervous system or post-infectious immune-mediated damage. This report presents a case of bilateral tremor of the upper limbs more than 6 weeks after a diagnosis of COVID-19, with confirmed volumetric brain loss shown by follow-up brain magnetic resonance imaging (MRI) combined with 3-dimensional volumetric NeuroQuant image analysis.
CASE REPORT: We report a case of new-onset tremors in a 62-year-old man after SARS-CoV-2 infection. MRI of the brain was performed shortly after the onset of tremors, and a follow-up MRI after 2 months showed evidence of rapid parenchymal volume, loss of midbrain substance, and increased cerebrospinal fluid volume within 2 months of the initial examination.
CONCLUSIONS: This case report shows central neurological effects of COVID-19, which can be evaluated by quantitative volumetric MRI analysis, although further studies are warranted to determine how this type of brain imaging can be used to evaluate the effects of SARS-CoV-2 infection over time.
Keywords: Atrophy, COVID-19, Mesencephalon, Movement Disorders, Tremor, Brain, Humans, Male, Middle Aged, SARS-CoV-2, Upper Extremity
Background
COVID-19 is associated with a range of neurological manifestations, including anosmia, headache, impaired consciousness, and stroke [1]. It is uncertain whether the SARS-CoV-2 directly targets the central nervous system, or whether the neurological symptoms are sequalae of a systemic or glymphatic response [2]. We report a case of a 62-year-old man with new-onset bilateral upper extremity tremors more than 6 weeks after SARS-CoV-2 infection, which was associated with brain volume loss atypical for the interval between the 2 magnetic resonance imaging (MRI) scans as measured by NeuroQuant software, the first FDA-cleared medical software for measuring volumes of brain structures and comparing the volumes to normative reference data adjusted according to age, sex, and intracranial volume [3].
Case Report
A 62-year-old man presented in March 2020 with cough, fever, and shortness of breath. He had a SARS-CoV-2 antigen test by nasal swab sampling using the Center for Disease Control reverse transcriptase-polymerase chain reaction primer test in an accredited laboratory at University Hospital, Columbia, Missouri; the result was positive. His social history was pertinent for working at a meat packing facility, where several of his coworkers had tested positive for COVID-19. After diagnosis, he self-quarantined at home for approximately 4 days, when he started experiencing worsening shortness of breath. He was admitted to our hospital for SARS-CoV-2 pneumonia and required intubation. He was successfully extubated on day 12 of admission and was discharged home in stable condition on day 19.
One month after hospital discharge, he began noticing new onsets of tremors. The tremors were progressive and present in the bilateral upper extremities, predominately in the fingers and wrists. Physician-directed personal interviews with the patient’s wife, work colleagues, family members, and primary care physician corroborated that the tremors were truly new onset after the SARS-CoV-2 infection. His other comorbidities were hypertension and type 2 diabetes mellitus. His medications included lisinopril, metformin, and amlodipine. There was no family history of tremors or history of illicit drug use.
A neurological examination confirmed that the patient had tremors in the bilateral distal upper extremities at the wrist, which were symmetric with an amplitude <0.1 cm on extended arm posture and increased when he was holding a fork near his mouth and while grabbing a cup. The tremor frequency was clinically observed to be 8 to 10 Hz, without dysmetria or dysdiadochokinesia. No rigidity or bradykinesia was noted. These tremors were not observed with rest. There was no associated tremor of the head, neck, or voice. There was no distractibility, entrainment or change in amplitude with motor coactivation (tapping with contralateral hand), and cognitive co-activation (serial subtraction of 7’s from 100). He denied any numbness, tingling, or weakness in any extremity. The neurological examination of the cranial nerves, muscle strength, deep tendon reflexes, and gait were unremarkable.
Laboratory tests in the form of complete blood count, comprehensive metabolic panel, vitamin B12, vitamin B6, vitamin E, venous lead level, thyroid stimulating hormone, free T4, ceruloplasmin level, creatine kinase, autoimmune and paraneoplastic antibodies, and ANA titers were within the reference range. We did not perform a spinal tap, as his history, presentation, and imaging did not raise any suspicion of meningitis, encephalitis, or any structural lesions warranting a lumbar puncture.
At the time of the patient’s hospitalization for SARS-CoV-2 infection, a 3T MRI (Siemens Healthineers) of the brain with and without contrast was performed and showed some minimal age-related white matter changes (Figure 1). Our MRI brain protocols include isotropic 3D 1-mm T1 magnetization-prepared rapid gradient echo and 1-mm isotropic 3D T2 fluid-attenuated inversion recovery imaging on all of our system scanners, which allow the neuroradiologists to make accurate intra-scanner comparisons. With the patient’s new onset clinical symptoms, a follow-up MRI with and without contrast was performed on a 1.5T MRI (Siemens Healthineers). The short-term follow-up MRI showed more prominent cerebral spinal fluid spaces, including the prepontine, suprasellar, basal cisterns, and Sylvian fissures, consistent with volume loss. The mid-line sagittal 3D T1 magnetization prepared rapid gradient echo (MP-RAGE) images of the midbrain at the level of the mammillary body showed interval subtle flattening of the mesencephalon on MRI after the patient’s onset of tremors (Figure 2). No significant abnormalities were noted in the basal ganglia, as shown in Figure 3.
To confirm our qualitative observation, we performed Siemens NeuroQuant volumetric brain analysis on the isotropic 3D T1 MP-RAGE series for both MRI examinations (Table 1). NeuroQuant is an automated FDA-cleared medical software that measures volumes of brain structures and compares the volumes to normative reference data adjusted according to age, sex, and intracranial volume [3,4]. We had previously confirmed the reproducibility of the brain analysis software across our MRI scanners. There was an increase of 34 mL (9.5%) in cerebral spinal fluid volume, with a reduction in total white matter of 36 mL (7.1%) on MRI 2 months after COVID-19. The volume of the third ventricle and mesencephalon did not show much difference from the initial volume (about 0.1 mL). The volumetric analysis confirmed our observation that there was an increase in cerebral spinal fluid volume and decrease in white matter volume at 2 months after COVID-19 and onset of tremors, with both beyond normal experimental errors. The volume of the cerebellum was within normal limits and no atrophy was noted on the volumetric analysis, as seen in Table 1.
Discussion
This case report presents a 62-year-old man with bilateral upper limb tremors more than 6 weeks after a diagnosis of COVID-19.
His volumetric analysis on MRI of the brain showed volume loss. To the best of our knowledge, analysis of volume loss has not been presented before. Various neurological manifestations have been reported with SARS-CoV-2 infections, including cranial nerve deficits, ischemic strokes, meningitis, delirium, intracerebral hemorrhages, encephalitis, and seizures [5]. Haddadi et al reported a case of altered mental status and bilateral basal ganglia hemorrhage as a presentation of SARSCoV-2 infection and hypothesized viral neuro-invasion as the most likely mechanism [6].
Cognitive complications such as dementia-like neurocognitive syndrome, affective disorders, psychosis, mental fatigue, and impaired concentration have also been reported [5]. Neurological manifestations are hypothesized to be due to direct effects of the virus on the central nervous system, hypoxic injury, and para-infectious or post infectious immune-mediated damage [1]. Solomon et al described post-mortem neuropathologic findings of 18 COVID-19-positive patients. Autopsy revealed hypoxic changes, while immunohistochemistry and RT-qPCR revealed minimal evidence of SARS-CoV-2 infection in different regions of the brain [7]. Gisslén et al measured plasma levels of glial fibrillary acidic protein and neurofilament light chain in 47 patients with COVID-19. In patients with severe COVID-19 (n=18), levels of both biomarkers were initially elevated; however, glial fibrillary acidic protein levels subsequently declined while neurofilament light chain levels continued to increase. Plasma glial fibrillary acidic protein levels were also raised in patients with moderate COVID-19 (n=9). These findings reflected a sequence of early astrocytic response and more delayed axonal injury and were thought to be due to immune activation linked to systemic SARS-CoV-2 infection or hypoxic/ischemic injury [8]. The ability of SARS-CoV-2 to penetrate the brain and infect the nervous system is also based on the high expression of angiotensin converting enzyme-2, which, after binding the SARS-CoV-2 virus, leads to internalization of the complex into the neuronal cell. This causes downregulation of the angiotensin converting enzyme receptor, resulting in an unopposed function of angiotensin II and decreased level of angiotensin (1–7). This imbalance may be partially responsible for the cytokine storm and subsequent damage. As angiotensin (1–7) binds to the Mas receptor and decreases inflammatory response, its decreased levels can also be a contributing factor for the hyper-inflammatory state [9].
Infectious diseases can be a potential factor causing secondary movement disorders in 20.4% of patients [10]. The development of transient parkinsonian symptoms has been reported in cases of viral infections such as avian flu, Epstein-Barr virus, Japanese encephalitis, West Nile virus, Coxsackie, and HIV. Possible mechanisms for neurological symptoms in these infections are induction of neuro-inflammation and hypoxic brain injury resulting in structural or functional damage within basal ganglia [11].
Brandao et al in a retrospective study identified 93 new-onset movement disorder cases and analyzed brain imaging in the form of head CT, brain MRI, and PET CT, revealing microbleeds, nigrosomal abnormality, and corpus splenium abnormalities in patients with tremor as clinical manifestation after COVID-19. However, the study did not include volumetric analysis of these specific structures [12].
Other potential causes of tremors have been ruled out by further investigations and corroboration by history, which includes no family history of tremors and onset after COVID-19. While the exact mechanism of tremors after COVID-19 remains unclear, we assume it to be multifactorial due to direct injury to the white matter tracts and pathways involving the midbrain from remnants of low titers of viral infection, which is supported by atypical rapid volume loss on MRI.
Conclusions
Our case demonstrates new-onset bilateral upper extremity tremors after COVID-19, which was associated with rapid loss of brain volume and increased cerebral spinal fluid spaces. This case report shows central neurological effects of COVID-19 that can be evaluated by quantitative volumetric MRI analysis. Neuronal loss can be potentially assessed after COVID-19 by quantitative volumetric analysis on a high-resolution, 1-mm isotropic voxel 3D T1 MP-RAGE series, where available. Vigilant and careful neuroradiological assessment is needed, and should be kept in mind since it appears that patients with COVID-19 are at additional risk for developing new neurodegenerative conditions at an earlier age. Further studies are warranted to determine how this type of brain imaging may be used to evaluate the effects of SARS-CoV-2 infection over time.
Figures
References:
1.. Ahmed MU, Hanif M, Ali MJ, Neurological manifestations of COVID-19 (SARS-CoV-2): A review: Front Neurol, 2020; 11; 518
2.. Tremblay ME, Madore C, Bordeleau M, Neuropathobiology of COVID-19: The role for glia: Front Cell Neurosci, 2020; 14; 592214
3.. Brewer JB, Fully-automated volumetric MRI with normative ranges: Translation to clinical practice: Behav Neurol, 2009; 21; 21-28
4.. Brewer JB, Magda S, Airriess C, Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease: Am J Neuroradiol, 2009; 30; 578-80
5.. Varatharaj A, Thomas N, Ellul MA, Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study: Lancet Psychiatry, 2020; 7(10); 875-82 [Erratum in: Lancet Psychiatry. 2020;7:875–82]
6.. Haddadi K, Ghasemian R, Shafizad M, Basal ganglia involvement and altered mental status: A unique neurological manifestation of coronavirus disease 2019: Cureus, 2020; 12(4); e7869
7.. Solomon IH, Normandin E, Bhattacharyya S, Neuropathological features of COVID-19: N Engl J Med, 2020; 383(10); 989-92
8.. Kanberg N, Ashton NJ, Andersson LM, Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19: Neurology, 2020; 95(12); e1754-59
9.. Mahmudpour M, Roozbeh J, Keshavarz M, COVID-19 cytokine storm: The anger of inflammation: Cytokine, 2020; 133; 155151
10.. Netravathi M, Pal PK, Indira Devi B, A clinical profile of 103 patients with secondary movement disorders: Correlation of etiology with phenomenology: Eur J Neurol, 2012; 19(2); 226-33
11.. Sulzer D, Antonini A, Leta V, COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside: NPJ Parkinsons Dis, 2020; 6; 18
12.. Brandão PRP, Grippe TC, Pereira DA, New-onset movement disorders associated with COVID-19: Tremor Other Hyperkinet Mov (N Y), 2021; 11; 26
Figures
Tables
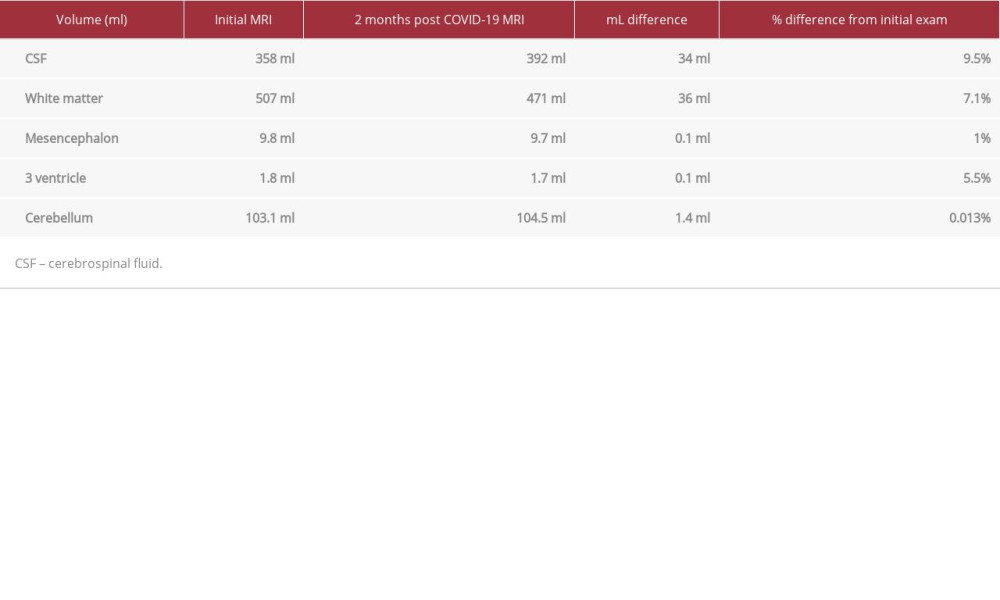 Table 1.. Volumes of cerebral spinal fluid, white matter, the mesencephalon, third ventricle, and cerebellum calculated by Siemens Healthineers brain volumetric analysis, NeuroQuant, shows significant short-term increased cerebral spinal fluid and decreased white matter volume, indicating volume loss.
Table 1.. Volumes of cerebral spinal fluid, white matter, the mesencephalon, third ventricle, and cerebellum calculated by Siemens Healthineers brain volumetric analysis, NeuroQuant, shows significant short-term increased cerebral spinal fluid and decreased white matter volume, indicating volume loss. Table 1.. Volumes of cerebral spinal fluid, white matter, the mesencephalon, third ventricle, and cerebellum calculated by Siemens Healthineers brain volumetric analysis, NeuroQuant, shows significant short-term increased cerebral spinal fluid and decreased white matter volume, indicating volume loss.
Table 1.. Volumes of cerebral spinal fluid, white matter, the mesencephalon, third ventricle, and cerebellum calculated by Siemens Healthineers brain volumetric analysis, NeuroQuant, shows significant short-term increased cerebral spinal fluid and decreased white matter volume, indicating volume loss. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








