25 June 2022: Articles 
Necrotizing Fasciitis in a Patient with Metastatic Clear Cell Ovarian Carcinoma Treated with Bevacizumab
Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Unexpected drug reaction
Asim Haider1ABCDEF*, Hitesh Gurjar1DEF, Haider Ghazanfar1DEF, Sridhar Chilimuri1ABCDEFDOI: 10.12659/AJCR.935584
Am J Case Rep 2022; 23:e935584
Abstract
BACKGROUND: Necrotizing fasciitis is a life-threatening infection of the deep soft tissues that leads to progressive destruction of the fascia and subcutaneous fat. It typically spreads along the muscle fascia planes because of the relatively poor blood supply. Muscle tissue is usually spared because of its better blood supply. The usual risk factors for necrotizing fasciitis include trauma, malnutrition, obesity, uncontrolled diabetes mellitus, alcoholism, cirrhosis, neutropenia, and recent surgery.
CASE REPORT: We present a case of a middle-aged female who presented with necrotizing fasciitis of the right gluteal region. Her medical history was significant for well-controlled diabetes mellitus (hemoglobin A1c: 6.6), and clear cell carcinoma of ovaries (stage IV). She was on active chemotherapy with bevacizumab, paclitaxel, and carboplatin. She underwent incision and debridement of right gluteal abscess with drainage of 200 ml of foul-smelling pus and was started on intravenous antibiotics. Her blood cultures were negative, but the cultures taken from the right gluteal abscess showed moderate growth of Escherichia coli. The antibiotics were de-escalated and the patient was discharged with outpatient follow-up.
CONCLUSIONS: Bevacizumab, a humanized monoclonal IgG antibody, is a novel treatment for metastatic ovarian cancer. It is associated with necrotizing fasciitis due to anti-angiogenic, pro-thrombotic, and poor wound healing properties. It should be stopped in the patients presenting with necrotizing fasciitis
Keywords: bevacizumab, Fasciitis, Necrotizing, Vascular Endothelial Growth Factors, Abscess, Anti-Bacterial Agents, Antibodies, Monoclonal, Humanized, Female, Humans, Middle Aged, Ovarian Neoplasms
Background
Bevacizumab is a humanized monoclonal IgG antibody. It is an inhibitor of vascular endothelial growth factor (VEGF). VEGF is a proangiogenic growth factor involved in tissue migration and proliferation and endothelial cell stabilization [1]. Therapies targeting VEGF are an important part of anticancer therapy [2]. The use of bevacizumab in advanced ovarian clear cell carcinoma has been shown to improve survival [3]. The common adverse effects of bevacizumab include thromboembolic events, proteinuria, hypertension, arthralgia, gastrointestinal perforation, and poor wound healing [4]. We present a case of necrotizing fasciitis of the right gluteal region in a patient on treatment with bevacizumab for metastatic clear cell carcinoma of the ovaries. This relationship has been rarely reported in the clinical setting.
Case Report
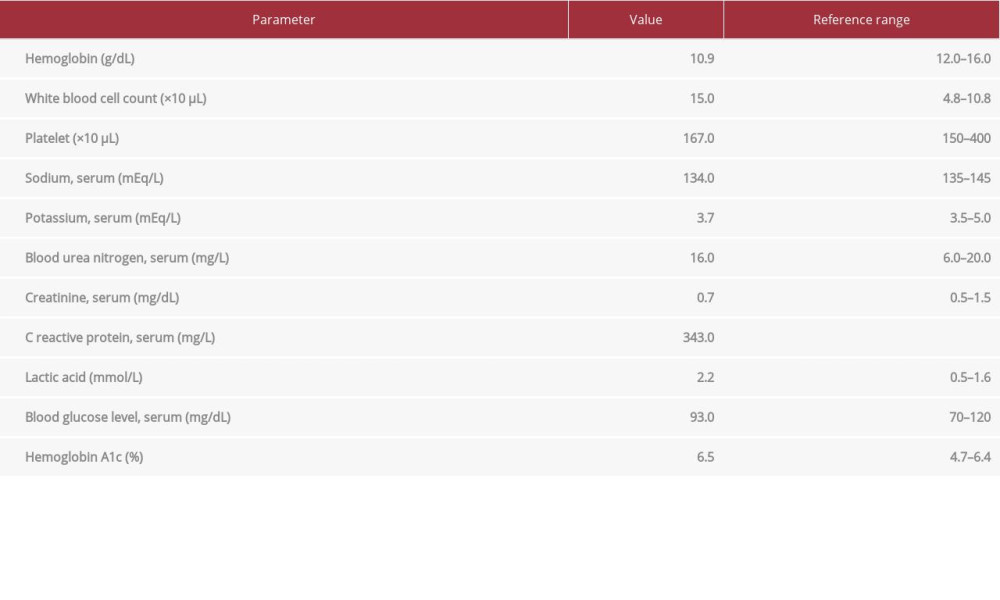
A 58-year-old woman had medical comorbidities of hypertension, diabetes mellitus (well-controlled, hemoglobin A1c: 6.6), obstructive sleep apnea on continuous positive airway pressure therapy, and clear cell carcinoma of the ovaries (stage IV), with abdominal lymph node involvement diagnosed in 2013. She underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy in November 2013. Surgery was followed by chemotherapy with carboplatin and paclitaxel every 3 weeks for 6 cycles from December 2013 to April 2014. In January 2019, she had an ovarian cancer recurrence with metastasis to the sigmoid colon, when she presented with rectal bleeding for a week. A partial colectomy with end-to-end anastomosis was performed. Adjuvant chemotherapy was planned, but the patient did not follow up for almost a year. Five months prior to presentation, she was started on chemotherapy with bevacizumab, paclitaxel, and carboplatin. She came to the Emergency Department with pain and swelling in the right gluteal region for 1 week. She reported that 1 week ago she started experiencing these symptoms associated with subjective feelings of fever and chills. She denied any radiation of the pain, neurologic deficits, bowel or bladder problems, change in bowel habits, urinary symptoms, or any vaginal/urethral discharge. She denied any history of smoking, alcohol, or drug abuse. On physical examination, her vital signs were as follows: heart rate, 135 beats per min; temperature, 38°C; blood pressure, 142/77 mmHg; and 96% oxygen saturation on room air. She was found to have a large fluctuant indurated mass in the lower right gluteal region, without any drainage or skin ulceration. Digital rectal examination revealed tenderness in the right rectal aspect, without any palpable mass. Initial laboratory results were significant for neutrophilic leukocytosis (neutrophils 83%, normal 40–70%), lactic acidosis, and elevated C-reactive protein. The laboratory values are summarized in Table 1. Her clinical course was further complicated by septic shock, which required the administration of low-dose norepinephrine for a short period of time. A computerized tomography scan of the pelvis with contrast material showed a right gluteal abscess of 7.4×4.1 cm, with an air-fluid level concerning for necrotizing fasciitis (Figure 1).
The patient underwent incision and debridement of the right gluteal abscess, with drainage of 200 mL of foul-smelling pus. She was admitted to the inpatient unit. Therapy with broad-spectrum antibiotics (intravenous [i.v.] vancomycin, i.v. piperacillin+tazobactam, and i.v. clindamycin) was started. Norepinephrine was gradually tapered off. The blood culture results were negative, but the cultures taken from the right gluteal abscess showed moderate growth of
Discussion
Necrotizing fasciitis is an infection of the deep soft tissues resulting in progressive destruction of the muscle fascia and the overlying fat tissue. The incidence of necrotizing fasciitis ranges from 0.3 to 15 cases per 100 000 [5]. Risk factors for necrotizing soft tissue infections include trauma, malnutrition, obesity, diabetes mellitus, alcoholism, cirrhosis, neutropenia, recent surgery, and use of sodium-glucose cotransporter 2 inhibitors [6]. Clinical manifestations of necrotizing infection include fever, severe pain (out of proportion to physical examination), erythema, crepitus, and skin bullae/ecchymosis [7].
Tumor cells and tissues are characterized by an increase in the metabolic rate and oxygen demands. The resulting tissue hypoxia leads to the production of VEGF, which binds to its receptors on the endothelial surface and leads to angiogenesis by stimulating the proliferation of endothelial cells [8]. The inhibition of VEGF via bevacizumab leads to a reduction in microvascular growth. At the same time, bevacizumab has been associated with an increase in arterial and venous thromboembolic events [9]. One meta-analysis conducted by Abdullah et al showed that patients who received bevacizumab were at significant risk for overall thromboembolic events, with a relative risk of 1.334 (95% CI, 1.191–1.494) [9]. The combined prothrombotic and antiangiogenic effects of bevacizumab are believed to cause tissue ischemia and necrosis, leading to necrotizing fasciitis [10]. Bevacizumab has also been associated with poor wound healing, which makes wounds susceptible to bacterial infection-causing necrotizing fasciitis [11].
In a study conducted to assess the safety of bevacizumab, 52 serious case reports of necrotizing fasciitis were identified, which occurred between November 1997 and September 2012 worldwide [12]. Health Canada issued a safety warning about the association of bevacizumab with necrotizing fasciitis [12]. Bevacizumab should be discontinued temporarily or permanently depending upon the severity of the adverse effects. In patients with moderate to severe proteinuria and uncontrolled hypertension, bevacizumab can be discontinued temporarily until the stabilization of the clinical condition. However, in a patient with gastrointestinal perforation, serious hemorrhage, thromboembolic phenomenon, nephrotic syndrome, necrotizing fasciitis, or hypertensive encephalopathy, therapy should be discontinued permanently [13].
It is difficult to establish a causal relationship between bevacizumab and necrotizing fasciitis in patients receiving chemotherapy who are already immunocompromised. However, since these patients are already immunosuppressed, it becomes even more important to explore this association, as shown in prior studies [12]. Similarly, although diabetes is associated with defects in innate and adaptive immunity, the clinical relevance of this effect is not well established in patients with good glycemic control (especially with hemoglobin A1c <8%) [14]. Our patient had a hemoglobin A1c level of 6.6%. The presence of peripheral artery disease in patients with diabetes increases the risk of necrotizing fasciitis. Our patient had no evidence of any macrovascular or microvascular complication related to diabetes mellitus. The development of necrotizing fasciitis as such has a unifying phenomenon of poor tissue perfusion, which when combined with inoculation of infectious agent, causes spread of a life-threatening infection [15]. Because bevacizumab is an angiogenesis inhibitor, it remains a potential factor in the development of such infections. Our patient did not have any preceding history of ulcer or trauma. A study that screened the US FDA Adverse Event Reporting System database to identify adverse events linked to bevacizumab found that necrotizing fasciitis was independently associated with bevacizumab use [16].
Conclusions
Bevacizumab is a novel treatment for metastatic ovarian cancer. It can cause necrotizing fasciitis owing to its antiangiogenic, prothrombotic, and poor wound healing properties. Early recognition with cessation of therapy is essential in the management of this complication, especially in patients who are already immunosuppressed and can have life-threatening outcomes.
References:
1.. Kazazi-Hyseni F, Beijnen JH, Schellens JH, Bevacizumab: Oncologist, 2010; 15(8); 819-25
2.. Mancuso MR, Davis R, Norberg SM, Rapid vascular regrowth in tumors after reversal of VEGF inhibition: J Clin Invest, 2006; 116(10); 2610-21
3.. Tate S, Nishikimi K, Matsuoka A, Bevacizumab in First-line chemo-therapy improves progression-free survival for advanced ovarian clear cell carcinoma: Cancers (Basel), 2021; 13(13); 3177
4.. Nalluri SR, Chu D, Keresztes R, Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis: JAMA, 2008; 300(19); 2277-85
5.. Stevens DL, Bryant AE, Necrotizing soft-tissue infections: N Engl J Med, 2017; 377(23); 2253-65
6.. Eneli I, Davies HD, Epidemiology and outcome of necrotizing fasciitis in children: An active surveillance study of the Canadian Paediatric Surveillance Program: J Pediatr, 2007; 151(1); 79-84.e1
7.. Anaya DA, Dellinger EP, Necrotizing soft-tissue infection: Diagnosis and management: Clin Infect Dis, 2007; 44(5); 705-10
8.. Mancuso MR, Davis R, Norberg SM, Rapid vascular regrowth in tumors after reversal of VEGF inhibition: J Clin Invest, 2006; 116(10); 2610-21
9.. Alahmari AK, Almalki ZS, Alahmari AK, Guo JJ, Thromboembolic events associated with bevacizumab plus chemotherapy for patients with colorectal cancer: A meta-analysis of randomized controlled trials: Am Health Drug Benefits, 2016; 9(4); 221-32
10.. Sendur MA, Aksoy S, Özdemir NY, Zengin N, Necrotizing fasciitis secondary to bevacizumab treatment for metastatic rectal adenocarcinoma: Indian J Pharmacol, 2014; 46(1); 125-26
11.. Zhang H, Huang Z, Zou X, Liu T, Bevacizumab and wound-healing complications: A systematic review and meta-analysis of randomized controlled trials: Oncotarget, 2016; 7(50); 82473-81
12.. , Avastin (bevacizumab) – cases of necrotizing fasciitis reported – for health professionals. Recalls and safety alerts April 29, 2013 Retrieved from https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/28921a-eng.php
13.. , Center for Drug Evaluation and Research Final Labeling Text, BL125085 Supplement, 2008. [cited 2010 February 15]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125085s0168lbl.pdf
14.. Peleg AY, Weerarathna T, McCarthy JS, Davis TM, Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control: Diabetes Metab Res Rev, 2007; 23(1); 3-13
15.. Stevens DL, Bryant AE, Goldstein EJ, Necrotizing soft tissue infections: Infect Dis Clin North Am, 2021; 35(1); 135-55
16.. Shamloo BK, Chhabra P, Freedman AN, Novel adverse events of bevacizumab in the US FDA adverse event reporting system database: A disproportionality analysis: Drug Saf, 2012; 35(6); 507-18
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250