04 March 2022: Articles 
Dramatic Response to Trastuzumab Deruxtecan Rechallenge in a Patient with HER2-Positive Gastric Cancer: A Case Report
Unusual setting of medical care
Takatsugu Ogata1ABCDEF, Yasuko FujitaDOI: 10.12659/AJCR.935600
Am J Case Rep 2022; 23:e935600
Abstract
BACKGROUND: Trastuzumab deruxtecan (T-DXd) has shown promising efficacy against human epidermal growth factor receptor 2 (HER2)-positive gastric and gastroesophageal junction (GEJ) adenocarcinomas. The efficacy of T-DXd rechallenge, however, has remained unclear. This is the first report of a dramatic response to T-DXd rechallenge in a patient with HER2-positive GEJ adenocarcinoma after confirmation of HER2 overexpression immediately prior to the rechallenge.
CASE REPORT: A 67-year-old man was diagnosed with HER2-positive gastric cardia (or GEJ) adenocarcinoma with lymph node and liver metastases. Initial T-DXd therapy was started as fourth-line chemotherapy. The best response was partial, and progression-free survival was 5.6 months. After an immune checkpoint inhibitor-based regimen, a rechallenge with T-DXd was planned as a seventh-line treatment. HER2 overexpression was confirmed by re-biopsy immediately before the rechallenge. He is currently receiving T-DXd without progression or severe treatment-related adverse events.
CONCLUSIONS: This is the first case report of a response to T-DXd rechallenge in a patient with HER2-positive gastric cancer. This rechallenge could be considered a treatment strategy for HER2-positive gastric cancer, for cases in which the initial T-DXd treatment was effective. Confirmation of HER2 overexpression and re-biopsy immediately before the rechallenge would be important for this strategy.
Keywords: Biopsy, Stomach Neoplasms, trastuzumab deruxtecan, Camptothecin, Humans, Immunoconjugates, Male, Trastuzumab
Background
According to the ToGA study, the first-line treatment for human epidermal growth factor receptor 2 (HER2)-positive gastric cancer is trastuzumab-based chemotherapy [1]. Other anti-HER2 therapies have also been reported for HER2-positive gastric cancer, but no study, except the ToGA study, has shown the superiority of anti-HER2 therapy [2–5]. Trastuzumab deruxtecan (T-DXd) is an antibody-drug conjugate composed of a monoclonal anti-HER2 antibody and topoisomerase I inhibitor. According to the DESTINY-Gastric01 study, T-DXd results in a more promising tumor response than the physician’s choice of chemotherapy [6]. Therefore, T-DXd was approved for the treatment of HER2-positive gastric cancer in Japan in September 2020. T-DXd has now become the standard third-line chemotherapy treatment for HER2-positive gastric cancer.
Although some new drugs have also been approved for gastric cancer in recent years, anti-HER2 therapies are the most effective for HER2-positive gastric cancer. In breast cancer, trastuzumab beyond progression is one of the standard therapies, according to the GBG26/BIG03-05 study [7]. However, the T-ACT study did not show the same efficacy of trastuzumab beyond progression for gastric cancer; hence, it was not considered a standard treatment [8]. The efficacies of trastuzumab rechallenge and T-DXd rechallenge in gastric cancer remain unclear. This is the first report of a dramatic response to T-DXd rechallenge in a patient with HER2-positive gastric cancer after the confirmation of HER2 overexpression immediately prior to T-DXd rechallenge.
Case Report
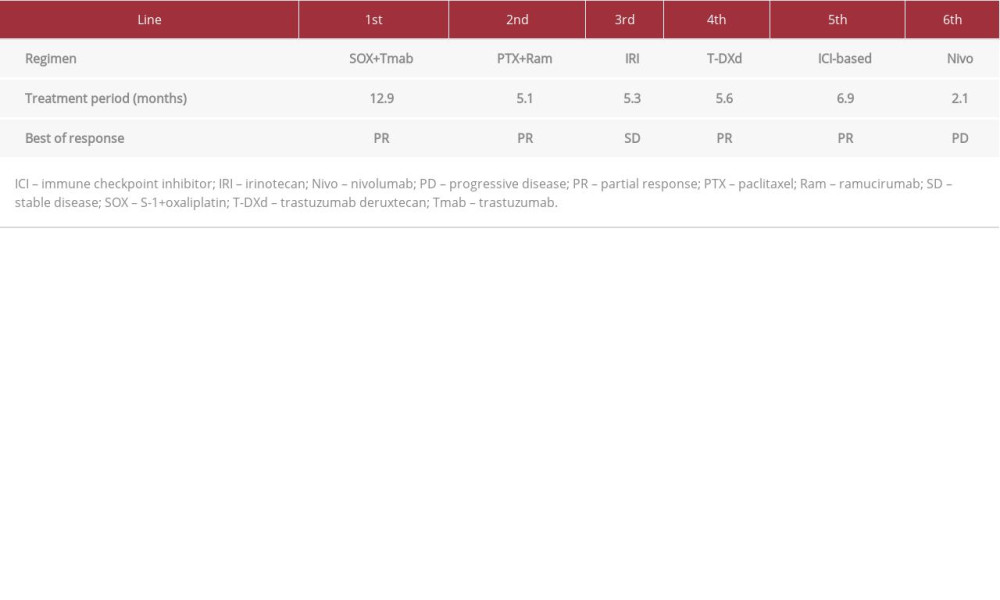
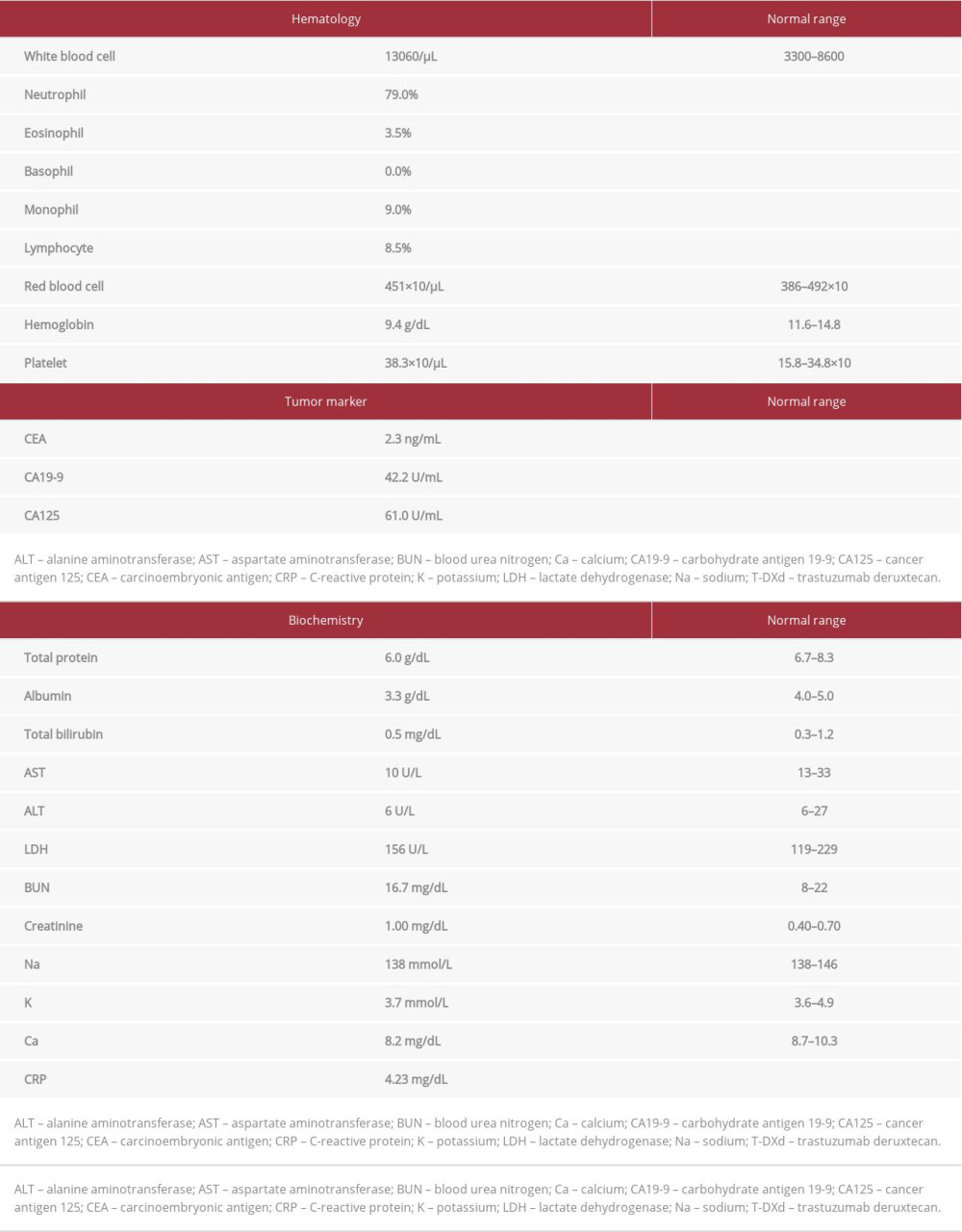
A 67-year-old male patient was diagnosed with gastric cardia cancer with lymph node and liver metastases. A biopsy of the primary gastric tumor was performed before first-line treatment, and a poorly differentiated adenocarcinoma was revealed. Immunohistochemistry (IHC; HercepTest, Dako, Denmark) showed the overexpression of HER2 (score=3) [9]. SOX plus trastuzumab therapy (100 mg/m2 oxaliplatin intravenous [IV], 6 mg/kg trastuzumab i.v. on day 1, and 80 mg/m2 S-1 p.o. on days 1-14, every 3 weeks) was started as the first-line treatment, and computed tomography (CT) imaging revealed that the disease had progressed even after 6 cycles. Paclitaxel plus ramucirumab therapy (80 mg/m2 paclitaxel i.v. on days 1, 8, and 15, and 8 mg/kg ramucirumab i.v. on days 1 and 15, every month) as the second-line treatment and irinotecan therapy (150 mg/m2 irinotecan i.v. on day 1, every 2 weeks) as the third-line treatment were administered, and the disease still progressed. He enrolled in a phase I trial of T-DXd, and T-DXd was started as the fourth-line treatment in August 2017. The initial dose of T-DXd was 5.4 mg/kg, and it was administered every 3 weeks. The best response was partial, and progression-free survival was 5.6 months. Subsequently, an immune checkpoint inhibitor was administered as the fifth-line and sixth-line treatments (Table 1). After the fifth-line treatment, the chemotherapy-free interval was 15.2 months because of sacral fracture due to osteoporosis. He reported difficulty swallowing during the treatment. CT and upper gastrointestinal endoscopy (UGE) revealed further progression of the primary site (Figures 1A, 2A). A re-biopsy of the primary gastric tumor was performed. IHC analysis revealed the overexpression of HER2 (score=2), and the biopsy specimen was positive on dual-color in-situ hybridization (INFORM HER2 Dual ISH DNA Probe Cocktail Assay; Figure 3A–3C). Rechallenge with T-DXd (6.4 mg/kg, every 3 weeks) was planned as the seventh-line treatment in March 2021. Laboratory data on the administration of T-DXd are summarized in Table 2. After the first cycle, the patient’s condition improved. After 3 cycles, CT showed a tumor response (Figure 1B). UGE showed shrinkage of the tumor at the primary site (Figure 2B) and HER2 overexpression was maintained after 4 cycles (Figure 3D–3F). The patient is currently receiving T-DXd without progression (Figure 1C). Treatment-related adverse events did not occur during treatment.
A gene panel test (FoundationOne® CDx, Foundation Medicine, USA) was performed, and genetic alterations were identified as follows: tumor mutational burden 18 Mut/Mb,
Discussion
Herein, we have reported the first case of a dramatic response to T-DXd rechallenge in a patient with HER2-positive gastric cancer, with HER2 positivity being confirmed immediately before the rechallenge. This case suggested that re-biopsy immediately before the T-DXd rechallenge was important, and in the case of HER2 overexpression, T-DXd rechallenge was helpful.
Some previous reports have indicated the loss of HER2 positivity after initial trastuzumab-based chemotherapy; the T-ACT study, the most recent study in Japan, demonstrated that HER2 positivity is maintained at only 31% after trastuzumab-based chemotherapy [8,10,11]. This study also showed an association between trastuzumab-free intervals and the efficacy of trastuzumab beyond progression. The data suggest that HER2 is re-expressed during trastuzumab-free chemotherapy. Three possible mechanisms underlie HER2 loss: (1) clonal selection, (2) heterogeneity of HER2 expression, and (3) use of fixatives other than 10% neutral buffered formalin [11]. Whether the loss of HER2 positivity occurred after T-DXd challenge remains unclear. In our case, although the expression of HER2 became weak after T-DXd challenge, the overexpression of HER2 was maintained.
In the DESTINY-Gastric01 study, the overall response rate (ORR) for T-DXd in gastric and GEJ adenocarcinoma with HER2 over-expression was significantly higher than that with chemotherapy (51.3% vs 14.3%,
In gastric and GEJ adenocarcinoma, biopsies are generally difficult to perform for patients with recurrent cancer after resection of the primary tumor. Anti-epidermal growth factor receptor (EGFR) antibodies are effective for patients
Recently, nivolumab and trifluridine/tipiracil have been approved for the treatment of gastric and GEJ adenocarcinoma. However, their response rate is only 4–11% [15–17], which is notably lower than that of T-DXd. The idea that the usefulness of T-DXd rechallenge can be determined by re-biopsy from the tumor and that the rechallenge would be meaningful if the tumor is HER2-positive could be of clinical importance.
Conclusions
We report the first case of response to T-DXd rechallenge in HER2-positive gastric and GEJ adenocarcinoma. Rechallenge with T-DXd could be a treatment strategy worth considering for HER2-positive gastric cancer for cases in which the initial T-DXd treatment was effective. Confirmation of HER2 over-expression immediately before T-DXd rechallenge would be important, and re-biopsy could be a helpful and appropriate procedure for HER2-positive gastric and GEJ adenocarcinoma before T-DXd rechallenge.
Figures
References:
1.. Bang YJ, Van Cutsem E, Feyereislova A, Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial: Lancet, 2010; 376; 687-97
2.. Tabernero J, Hoff PM, Shen L, Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study: Lancet Oncol, 2018; 19; 1372-84
3.. Hecht JR, Bang YJ, Qin SK, Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adeno-carcinoma: TRIO-013/LOGiC – a randomized phase III trial: J Clin Oncol, 2016; 34; 443-51
4.. Satoh T, Xu RH, Chung HC, Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN – a randomized, phase III study: J Clin Oncol, 2014; 32; 2039-49
5.. Thuss-Patience PC, Shah MA, Ohtsu A, Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study: Lancet Oncol, 2017; 18; 640-53
6.. Shitara K, Bang YJ, Iwasa S, Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer: N Engl J Med, 2020; 382; 2419-30
7.. von Minckwitz G, Schwedler K, Schmidt M, Trastuzumab beyond progression: Overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer: Eur J Cancer, 2011; 47; 2273-81
8.. Makiyama A, Sukawa Y, Kashiwada T, Randomized, phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study): J Clin Oncol, 2020; 38; 1919-27
9.. Rüschoff J, Dietel M, Baretton G, HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing: Virchows Arch, 2010; 457; 299-307
10.. Seo S, Ryu MH, Park YS, Loss of HER2 positivity after anti-HER2 chemo-therapy in HER2-positive gastric cancer patients: Results of the GASTric cancer HER2 reassessment study 3 (GASTHER3): Gastric Cancer, 2019; 22; 527-35
11.. Saeki H, Oki E, Kashiwada T, Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604): Eur J Cancer, 2018; 105; 41-49
12.. Yamaguchi K, Bang YJ, Iwasa S, 1422MO trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-low, advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Results of the exploratory cohorts in the phase II, multicenter, open-label DESTINY-Gastric01 study: Ann Oncol, 2020; 31; S899-900
13.. Shitara K, Bang Y, Iwasa S, O-14 exploratory biomarker analysis of trastuzumab deruxtecan in DESTINY-Gastric01, a randomized, phase 2, multicenter, open-label study in patients with HER2-positive or -low advanced gastric or gastroesophageal junction adenocarcinoma: Ann Oncol, 2021; 32; S224
14.. Sartore-Bianchi A, Pietrantonio F, Lonardi S, Phase II study of anti-EGFR rechallenge therapy with panitumumab driven by circulating tumor DNA molecular selection in metastatic colorectal cancer: The CHRONOS trial: J Clin Oncol, 2021; 39; 3506-14
15.. Kang YK, Boku N, Satoh T, Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial: Lancet, 2017; 390; 2461-71
16.. Shitara K, Doi T, Dvorkin M, Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial: Lancet Oncol, 2018; 19; 1437-48
17.. Kito Y, Inoue E, Akamaru Y, Survival analysis by tumor response from real-world data in advanced gastric cancer treated with nivolumab: The DELIVER trial (JACCRO GC-08): J Clin Oncol, 2021; 39; 4044
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250