28 April 2022: Articles 
Use of Lithium in Hyperthyroidism Secondary to Graves’ Disease: A Case Report
Unusual clinical course, Challenging differential diagnosis, Diagnostic / therapeutic accidents, Unusual setting of medical care, Unexpected drug reaction
Pranjali P. Sharma1ABDEF*DOI: 10.12659/AJCR.935789
Am J Case Rep 2022; 23:e935789
Abstract
BACKGROUND: The therapeutic approach to Graves’ disease (GD) comprises thionamides, radioiodine ablation, or surgery as first-line therapy, and cholestyramine and oral iodine as second-line therapies. The role of lithium (Li) in GD as a primary or adjunctive therapy remains contentious. We present a case of GD managed by Li therapy with oral iodine solution.
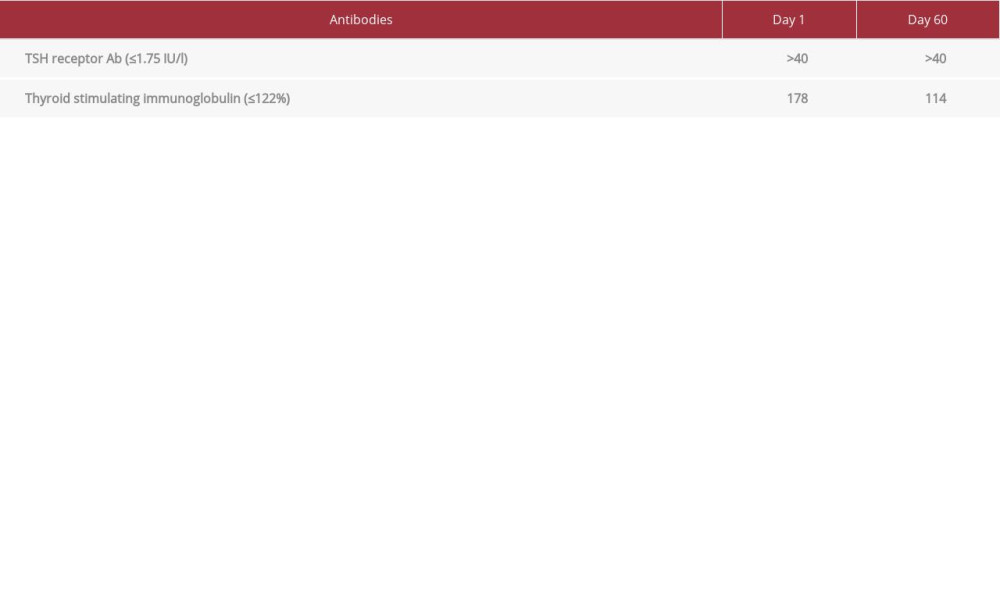
CASE REPORT: A 26-year-old man, admitted with acute blast crisis secondary to chronic myeloid leukemia (CML), reported palpitations, 40-lb weight loss, heat intolerance, and fatigue. An examination revealed sinus tachycardia, elevated body temperature, and thyromegaly. Laboratory evaluation confirmed hyperthyroidism (TSH <0.005 mcIU/l, FT4 5.57 ng/dl, TT3 629 ng/dl) secondary to GD (TRAb >40 IU/l, TSIg 178%). Thionamides and surgery were contraindicated due to pancytopenia from a blast crisis. Inability to maintain post-radiation precautions precluded use of RAI. Cholestyramine was attempted and discontinued due to nausea. We introduced oral Li carbonate with oral iodine, which the patient tolerated. Thyroid functions improved with therapy (TSH 0.007 mcIU/l, FT4 0.82 ng/dl, TT3 122 ng/dl) with stable Li level (0.5-0.8 mmol/l).
CONCLUSIONS: Li inhibits iodine uptake through interference with sodium-iodide symporter and tyrosine iodination, thyroglobulin structure changes, peripheral deiodinase blockage, and preventing TSH and TSIg stimulation. Our case shows that a low therapeutic level of Li, in combination with oral iodine, can suppress thyroid overactivity without adverse effects. We suggest that low-dose Li carbonate is a safe and effective adjunctive antithyroid medication to be considered if primary therapies for hyperthyroidism are unavailable.
Keywords: Graves’ disease, hyperthyroidism, Lithium Carbonate, Pancytopenia, Adult, Blast Crisis, Carbonates, Cholestyramine Resin, Graves Disease, Humans, Iodine, Iodine Radioisotopes, Lithium, Male, thyrotropin
Background
First-line therapeutic approaches to hyperthyroidism secondary to Graves’ disease (GD) comprise symptom control with beta-blocker and decreasing thyroid hormone synthesis through thionamides (antithyroid drugs, ATDs), RAI, or surgery [1]. Methimazole and propylthiouracil (PTU) are the most common ATDs used in the United States. Relatively mild adverse effects from ATDs include pruritus, rash, urticaria, arthralgias, arthritis, nausea, vomiting, or abnormal taste, occurring in up to 13% patients [2]. Serious adverse effects include agranulocytosis, hepatotoxicity, and vasculitis [1]. Agranulocytosis is more likely to occur with any dose of PTU than with low-dose methimazole and can be life-threatening [1], while it is dose-dependent with methimazole [3]. It usually occurs within the first 2–3 months of therapy; however, the overall incidence is relatively low, at 0.1–0.5% [1]. Occurrence of agranulocytosis with either PTU or methimazole is a contraindication to the use of alternate ATDs due to cross-reactivity [1]. Therefore, one has to consider alternate therapy options. Second-line adjunctive therapies include cholestyramine, oral iodine solutions, and glucocorticoids [1,4]. Rarely, lithium (Li) has been considered as an adjunct due to its thyroid hormone-lowering effect [5–7]. Its role, however, remains contentious.
Case Report
A 26-year-old man was admitted with acute blast crisis secondary to chronic myeloid leukemia (CML). He reported palpitations, 40-lb weight loss, heat intolerance, and fatigue, which he had attributed to CML. Examination revealed sinus tachycardia with heart rate of 120–140/min, hypertension with systolic reading of 140–160 mmHg, elevated body temperature of 37–39°C, and a prominent thyroid gland, without visual abnormalities. The patient did not have any signs of cognitive or gastrointestinal dysfunction. He did not have shortness of breath or pedal edema. Based on the Burch-Wartofsky scoring system for thyroid storm, the patient scored up to 40 points, suggestive of but not confirmatory for thyroid storm clinically. Laboratory evaluation on Day 1 of admission confirmed hyperthyroidism with TSH <0.005 mcIU/l (range 0.45–4.5 uIU/ml), free T4 (FT4) 5.57 ng/dl (range 0.82–1.77 ng/dl), total T3 (TT3) 629 ng/dl (60–180 ng/dl) (Figure 1) secondary to GD (TRAb >40 IU/l (range 0.0–1.75 IU/L), and TSIg 178% (range <140% baseline) (Table 1). Propranolol 40 mg 4 times a day was initiated for symptom control. ATDs and thyroidectomy were ruled out due to concerns of high morbidity and mortality from pancytopenia from the blast crisis. The patient was a prisoner and the prison would be unable to provide the post-radiation precautions (eg, social distancing, separate utensils for meals, and separate bathroom) required. Therefore, we were unable to proceed with RAI. The patient was prescribed cholestyramine 4 gm up to 3 times a day, which he only took for 1 day, after which he refused the medication due to excessive nausea and vomiting. Utilization of SSKI alone was considered but not pursued due to concern for worsening hyperthyroidism and precipitation of thyroid storm. Finally, after 2 weeks of inpatient hospital stay, oral Li carbonate 300 mg 3 times a day, with SSKI 50 mg/drop 1 drop 3 times a day, was introduced. Li levels were monitored every 2–3 days. Two weeks into treatment, FT4 and TT3 dropped to 4.51 ng/dl and 359 ng/dl, respectively. The patient tolerated Li without adverse effects. He reported nausea with the SSKI solution, but was willing to continue taking it. At hospital discharge 1 month later, thyroid tests showed improvement (TSH 0.007 mcIU/l, FT4 0.82 ng/dl, TT3 122 ng/dl) (Figure 1) with stable Li level (within 0.5–0.8 mmol/l) (Figure 2). The patient was sent back to prison and, due to miscommunication, Li carbonate and SSKI were discontinued by the team there. A month later (Day 60), at hospitalization for additional CML therapy, the patient’s thyroid tests showed hyperthyroidism (TSH <0.005 mcIU/l, FT4 3.67 ng/dl, TT3 235 ng/dl) and undetectable Li level (<0.2 mmol/l). Due to previous efficacy, Li carbonate and SSKI were restarted, with good biochemical response after 8 days (FT4 2.15 ng/dl, TT3 167 ng/dl) with low Li level (0.3 mmol/l). Medications were held during the patient’s chemotherapy for CML due to nausea and vomiting, following which, thyroid functions testing showed biochemical hyperthyroidism on Day 75 (TSH <0.005 mcIU/l, FT4 4.56 ng/dl, TT3 286 ng/dl) with undetectable Li levels (<0.2 mmol/l). When he felt better, Li and SSKI were restarted, with ongoing good response. The patient was advised to continue both medications and to follow up with endocrinology in the outpatient setting. So far, he remains on oral Li carbonate 300 mg 3 times a day and SSKI 50 mg/drop 1 drop 3 times a day, without adverse effects. Most recent laboratory test results continue to show an undetectable TSH, but FT4 and TT3 are within normal range (1.42 ng/dl and 98 ng/dl, respectively). The ultimate plan for this patient is a total thyroidectomy when his cell counts improve.
Discussion
Li carbonate has long been used for treatment of bipolar disorder and manic-depressive disorders [8]. Hypothyroidism and goiter were noted in patients treated with Li, leading to more investigation regarding the mechanisms.
Li affects cell function by inhibitory action on ATPase, cAMP, and inositol phospholipid metabolism. In vitro, it decreases the response of cultured cells to thyrotropin-releasing hormone (TRH) [9]. The thyroid gland is capable of Li uptake and concentration. It then inhibits iodine uptake through sodium-iodide symporter interference and interferes with tyrosine iodination [9]. Li promotes the conversion of thyroxine to triiodothyronine in the thyroid gland [8]. It decreases colloid droplet formation within the thyroid follicular cells [10]. Changes in thyroglobulin structure are seen, likely due to impaired proteolytic digestion [10]. These mechanisms prevent thyroid hormone release. Data have also shown that Li affects the hypothalamic-pituitary-thyroid axis through alteration in thyroid hormone receptor in the hypothalamus and brain deiodinase enzymes [9]. Li blocks deiodinases in the periphery and prevents TSH or stimulating antibodies from stimulating cells via the hormone receptors [11,12]. This shows that Li plays a multifactorial role in lowering thyroid hormone levels. These effects tend to be reversible and resolve after discontinuation of Li [13]. The reversible and diverse mechanism of action of Li was the primary reason for utilization of Li in our patient, who could not tolerate primary therapy.
Use of iodine for acute treatment of hyperthyroidism is well-known. Iodine acutely inhibits its own uptake, oxidation, and thyroid hormone secretion [14]. This is termed as the Wolff-Chaikoff effect. However, this effect is transient and, ultimately, there is escape, leading to hyperthyroidism. Therefore, adjuvant therapy is essential to prevent escape and worsening hyperthyroidism. The role of Li in enhancing the efficacy of RAI ablation for hyperthyroidism through inhibition of iodine release without preventing RAI uptake has been described [15]. Thus, Li increases the half-life of the RAI, thereby prolonging the dose delivery. A recent meta-analysis of 6 randomized controlled trials demonstrated better cure rates of hyperthyroidism when Li was used as an adjunct to RAI [16]. The authors also showed, in a subgroup analysis, that adding Li to RAI therapy in Graves’ disease, led to 15% higher cure rates as compared to RAI alone.
From a dose standpoint, studies evaluating role of Li in hyperthyroidism have utilized doses of 500–900 mg daily [16]. These doses are much lower than those used to treat bipolar or psychotic disorders. Our patient’s daily dose of 900 mg was on the higher end of the doses studied in the literature so far. Studies have also suggested that a short course of Li (around 7 days) after administration of RAI dose is most beneficial for RAI [16,17]. The immediate improvement in thyroid function testing in our patient with the combination of Li and SSKI confirms that Li has an additive effect with SSKI. The combination also allowed for utilization of less SSKI for more a prolonged period of time without precipitating hyperthyroidism through the escape phenomenon.
Li takes about 4 days to reach steady-state concentration [12]. Due to Li’s narrow therapeutic range (0.8–1.2 mmol/l), it is essential to monitor Li levels closely during therapy. Lithium toxicity occurs at levels above 1.5 mmol/l. Acute toxicity can present as gastrointestinal (nausea, vomiting, diarrhea), neurological (sluggishness, ataxia, confusion, tremors, fasciculations), or cardiac (QTc prolongation) symptoms. Chronic toxicity can precipitate polyuria or polydipsia to nephrogenic diabetes insipidus [13]. Our patient had nausea and vomiting, but these were attributed to ongoing chemotherapy for CML. Additionally, since the Li levels did not go above 0.8 mmol/l, it is unlikely that his symptoms were Li-related. Other adverse effects were not seen, even after several weeks of Li therapy.
Our case provides evidence of the utility of Li carbonate in hyperthyroidism as an adjunctive medication to oral iodine. We were able to show that a low therapeutic level of Li is enough to suppress thyroid overactivity without significant adverse effects. Our patient did not achieve Li levels above 0.8 mmol/l with improvement in thyroid function testing. The effects of Li on the thyroid proved to be reversible in our patient. Hence, medication compliance with periodic blood work for thyroid and Li levels is important. This case report does not provide enough support for use of Li carbonate as a primary or stand-alone therapy for hyperthyroidism secondary to GD. This will require a large-scale randomized control trial.
Conclusions
We suggest that Li carbonate is an effective oral adjunctive antithyroid medication that can be used to treat hyperthyroidism secondary to GD when first-line therapeutic options are not available. Whether Li carbonate is an effective antithyroid drug when used alone remains to be seen.
References:
1.. Ross DS, Burch HB, Cooper DS, 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis: Thyroid, 2016; 26(10); 1343-21 [Erratum in: Thyroid. 2017;27(11):1462]
2.. Sundaresh V, Brito JP, Wang Z, Comparative effectiveness of therapies for Graves’ hyperthyroidism: A systematic review and network meta-analysis: J Clin Endocrinol Metab, 2013; 98(9); 3671-77
3.. Takata K, Kubota S, Fukata S, Methimazole-induced agranulocytosis in patients with Graves’ disease is more frequent with an initial dose of 30 mg daily than with 15 mg daily: Thyroid, 2009; 19(6); 559-63
4.. Kaykhaei MA, Shams M, Sadegholvad A, Low doses of cholestyramine in the treatment of hyperthyroidism: Endocrine, 2008; 34(1–3); 52-55
5.. Nayak B, Burman K, Thyrotoxicosis and thyroid storm: Endocrinol Metab Clin North Am, 2006; 35(4); 663-86
6.. Lazarus JH, Lithium and thyroid: Best Pract Res Clin Endocrinol Metab, 2009; 23(6); 723-33
7.. Kessler L, Palla J, Baru JS, Lithium as an adjunct to radioactive iodine for the treatment of hyperthyroidism: A systematic review and meta-analysis: Endocr Pract, 2014; 20(7); 737-45
8.. Zheng R, Liu K, Chen K, Lithium carbonate in the treatment of Graves’ disease with ATD-induced hepatic injury or leukopenia: Int J Endocrinol, 2015; 2015; 694023
9.. Lazarus JH, The effects of lithium therapy on thyroid and thyrotropin-releasing hormone: Thyroid, 1998; 8(10); 909-13
10.. Williams JA, Berens SC, Wolff J, Thyroid secretion in vitro: inhibition of TSH and dibutyryl cyclic-AMP stimulated 131-I release by Li+1: Endocrinology, 1971; 88(6); 1385-88
11.. Terao T, Oga T, Nozaki S, Possible inhibitory effect of lithium on peripheral conversion of thyroxine to triiodothyronine: A prospective study: Int Clin Psychopharmacol, 1995; 10(2); 103-5
12.. Prakash I, Nylen ES, Sen S, Lithium as an alternative option in Graves thyrotoxicosis: Case Rep Endocrinol, 2015; 2015; 869343
13.. Gitlin M, Lithium side effects and toxicity: Prevalence and management strategies: Int J Bipolar Disord, 2016; 4(1); 27
14.. Chung HR, Iodine and thyroid function: Ann Pediatr Endocrinol Metab, 2014; 19(1); 8-12
15.. Bogazzi F, Bartalena L, Brogioni S, Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism: J Clin Endocrinol Metab, 1999; 84(2); 499-503
16.. Ahmed FW, Kirresh OZ, Majeed MS, Meta-analysis of randomized controlled trials comparing the efficacy of radioactive iodine monotherapy versus radioactive iodine therapy and adjunctive lithium for the treatment of hyperthyroidism: Endocr Res, 2021; 46(4); 160-69
17.. Sekulić V, Rajić M, Vlajković M, The effect of short-term treatment with lithium carbonate on the outcome of radioiodine therapy in patientswith long-lasting Graves’ hyperthyroidism: Ann Nucl Med, 2017; 31; 744-51
Figures
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250