19 April 2022: Articles 
A 55-Year-Old Man with Recurrent Gastrointestinal Bleeding Due to Stricture of the Portal Vein Anastomotic Site 12 Years After Combined Pancreas and Kidney Transplantation
Challenging differential diagnosis, Rare disease
Steffen Rassow12ABCDEF, Stefan BüttnerDOI: 10.12659/AJCR.936148
Am J Case Rep 2022; 23:e936148
Abstract
BACKGROUND: Varices of the upper gastrointestinal tract are due to portal hypertension and can result from occlusion of the portal venous system. This report is of a 55-year-old man with recurrent gastrointestinal bleeding due to stricture of the portal vein anastomotic site to inferior vena cava (IVC) 12 years after combined pancreas and kidney transplantation.
CASE REPORT: A 55-year-old man presented bleeding episodes requiring transfusion of more than 70 units of red blood cells (RBCs), complicated by bacterial and viral infection episodes including cytomegalovirus (CMV) reactivation and hepatitis E and transient impairment of function of the renal allograft. Endoscopy, computed tomography (CT) scan, and angiography revealed jejunal varices due to anastomotic stricture at the portal vein to IVC as the cause of the hemorrhage. Neither conservative therapy nor an anastomosis between the splenic vein of the graft and the internal iliac vein as a bypass could stop the life-threatening bleeding. During the recurrent bleeding, CD4 T lymphocytes were low, indicating immunodeficiency despite paused immunosuppressive therapy. After the hemorrhage resolved and immunosuppression was restarted, CD4 T lymphocyte levels normalized. Finally, to stop the hemorrhage and save the transplanted kidney and the patient’s life, graft pancreatectomy was performed. Long-term damage to the renal transplant was not found.
CONCLUSIONS: This report is of a rare case of portal hypertension as a long-term complication of transplant surgery. Although acute venous thrombosis at the anastomotic site is a recognized postoperative complication of pancreatic transplant surgery, this case highlights the importance of post-transplant follow-up and diagnostic imaging.
Keywords: Constriction, Pathologic, Gastrointestinal Hemorrhage, Kidney Transplantation, Pancreas Transplantation, Varicose Veins, Humans, Hypertension, Portal, Male, Pancreas, Portal Vein
Background
Simultaneous pancreas-kidney transplantation (SPKT) is the treatment of choice for patients with end-stage renal disease due to type 1 diabetes mellitus [1]. Moreover it is the most effective treatment for patients with type 1 diabetes mellitus and end-stage renal disease. SPKT increases patient survival, enhances quality of life, and prevents progression of diabetic complications [2]. Perioperative bleeding complications are not uncommon. Luminal bleeding complications can result from mucosal irritation, erosions, and ulcerations caused by infection or rejection [3]. Early transplant complications also include thrombosis and pancreatic fistula [2]. Causes of late bleeding in enteric-drained recipients described in the literature included ischemic duodenal ulcers, duodenal cytomegalovirus (CMV) infection, pancreatitis with erosion hemorrhage, arterial bleeding from ruptured pseudoaneurysm, or bleeding from the duodenojejunal enteric anastomosis [4–6]. The presence of periduodenal varices is an uncommon but a dangerous complication [7]. We then discuss case reports of jejunal varices in patients without SPKT and 2 cases of jejunal varices after SPKT. This report is of a 55-year-old man with recurrent gastrointestinal bleeding due to stricture of the portal vein anastomotic site 12 years after combined pancreas and kidney transplantation.
Case Report
A 55-year-old man presented with recurrent gastrointestinal bleeding episodes 12 years after simultaneous pancreas-kidney transplantation. This study was exempted from Institutional Review Board approval. Informed consent was obtained from the patient before publishing this case report, including images. In 2019 the patient was transferred to our institution from another hospital because of gastrointestinal bleeding of unknown origin with weakness and concomitant urinary tract infection with
The patient, who had received simultaneous transplantation of pancreas and kidney in 2007 due to diabetes type 1 with end-stage renal disease, had a medical history of stroke and coronary artery disease. Before transplantation, dialysis was started at the age of 42 years with a duration of 1.5 years. The pancreas graft was anastomosed with arterial anastomosis of a donor-iliac artery graft to the recipient common iliac artery, portal vein anastomosis to the inferior vena cava, and enteric exocrine drainage via side-to-side duodeno-jejunostomy. Following surgery, the course was uncomplicated with primary graft function (Pancreas: cold ischemia time (CIT) 10 h 28 min, warm ischemia time (WIT) 30 min; Kidney: CIT 10 h 28 min, WIT 50 min). Immunosuppression was started with tacrolimus, mycophenolate mofetil, and prednisolone and anti-thymocyte globulin as induction therapy. During follow-up, no rejection or infection episodes were noted.
After transferring the patient to our institution due to recurring hemorrhages, the function of the pancreas graft was normal, with euglycemia (C-peptide 7.9 ng/ml). The renal allograft function was initially impaired (serum creatinine 2.1 mg/dl) due to concomitant urinary tract infection. After anti-infective therapy with imipenem, renal function normalized. As gastrointestinal bleeding persisted, esophagogastroduodenoscopy (EGD) was repeated and showed an ulcer at the jejuno-duodenal anastomosis without any signs of bleeding, but there were varicose vessels (Figure 1) in the same area. CT angiography showed unsuspicious arterial and venous vessels and no signs of abdominal bleeding. Pantoprazole therapy was administered. Recurring low hemoglobin values (hemoglobin 7.0 g/dl) were treated by multiple transfusions of RBCs. During the clinical course, further tests such as a repeated esophagogastroduodenoscopy, including double-balloon endoscopy, colonoscopy, and capsule endoscopy, were all negative for another acute bleeding focus. The result of a bone marrow biopsy that was performed for low peripheral leucocyte counts showed no abnormalities. Moreover, the patient had recurrent septic episodes with impairment of renal function. Thus, immunosuppressive medication was reduced and transiently stopped during episodes of severe infections, especially as the CD4-positive T lymphocyte count (196/µl) was low, which suggests immunodeficiency at that time; low-dose hydrocortisone treatment to prevent adrenal insufficiency in a long-term steroid-treated patient was given instead of prednisolone. During the complicated course, liver function was impaired with elevated liver enzymes alanine transaminase and aspartate transaminase (ALAT and ASAT) (Figure 2A, 2B). An abdominal ultrasound showed no signs of cirrhosis. Complicating the recurrent hemorrhage was a new positive finding of hepatitis E infection with high viral loads concomitant with ascites and CMV reactivation in the blood.
The patient’s health was deteriorating and 2 months after admission he developed hemorrhagic shock.
During the hemorrhagic shock, another EGD was performed, jejunal varices were clipped, and an adrenalin injection was performed, but the hemorrhage could not be stopped. A new CT scan showed a stenosis of the porto-caval anastomosis (Figure 3); therefore, a venous angiography via the right superior femoral vein was performed (5F sidewinder catheter). The stenosis at the location of the anastomosis between inferior vena cava (IVC) and portal vein of the pancreas graft was dilated with a balloon catheter (8×40 mm) (Figure 4A–4C). Narrowing of the vena cava was ruled out. The partial improvement of the stenosis was proven a few days later by CT scan (Figure 5). Perfusion of the abdominal organs e.g. the liver and pancreas graft was unremarkable. However, the need for erythrocyte transfusions continued and the patient had recurrently low hemoglobin levels during acute bleeding episodes. During the whole time there were no cardiovascular adverse events, and normal ejection fraction with normal chambers of the heart were proven by echocardiography.
After 3 months of continuing recurrent bleeding events, a surgical intervention was undertaken by performing an end-to-side anastomosis of the splenic vein of the pancreas graft at the tail of the pancreatic graft with the right iliac vein of the recipient (analogous to a splenorenal shunt in portal hypertension). During surgery, the pancreas graft appeared unremarkable macroscopically. Few adhesions were noted. Tortuous collateral vessels were present between donor’s duodenum and the adjacent parts of the intestine. The right internal iliac vein was mobilized in an antero-lateral direction for the new anastomosis. After performing the new anastomosis between the splenic vein and internal iliac vein with a bovine patch for extension, the anastomosis was patent and it appeared that decompression of the splenic and portal vein of the pancreas graft had been accomplished. Color-coded duplex ultrasound verified a retrograde perfusion of the splenic vein with a diameter of about 3 to 4 millimeters. Thus, it seemed that decompression of the venous outflow of the pancreatic graft caused by late stenosis of the portocaval anastomosis had been relieved. Unfortunately, blood transfusions were still needed because of low hemoglobin values. After 10 more days in the Intensive Care Unit (ICU), considering the clinical course with the abovementioned interventions and uncontrollable recurring bleeding, the decision for graft pancreatectomy was made to save the kidney transplant. A part of the small intestine and the donor pancreas were resected. No complications were noted during the pancreatectomy. Next to the chronic inflammation of the resected tissues, histologically vessels of the bigger lumina were found as evidence of the intestinal tortuous collateral vessels. Finally the bleeding was stopped after transfusing more than 70 units of RBCs in total during hospitalization. For 5 months after the pancreatic allograft removal, throughout the follow-up, no more blood products were needed. In general, the patient’s health improved. Hepatitis E infection could no longer be detected. CMV reactivation was treated successfully by ganciclovir, and the CD4 T cell count recovered to nearly normal range. After 3 months without immunosuppressive medication, a regimen with tacrolimus and prednisolone was started again to provide adequate immunosuppression for the renal allograft, which was still in-situ with good function with a serum creatinine of 1.2 mg/dl. In our case there were no findings of donor-specific human leukocyte antigen-antibodies in a single-antigen bead assay. After 180 days in hospital, the patient was discharged in good health. The patient’s health and kidney function remain good over 12 months of follow-up.
Discussion
Mid-intestinal bleeding because of jejunal varices is a rare cause of gastrointestinal hemorrhage. Diagnostics and therapy can be challenging. Long-term follow-up, especially after pancreas-kidney transplantation, is important.
Diagnosis is challenging, as it was in our case. For diagnosis and treatment, good interdisciplinary cooperation was needed. In our patient, jejunal varices were intended to be treated with a clip application in EGD. Injection of Histoacryl did not prevent the development of the varices. Angiographic embolization was not tried due to poor venous drainage with a radiologically-proven stenosis at the porto-systemic venous anastomosis as the cause of the jejunal varices. Late stenosis of the anastomosis between the graft portal vein and recipient IVC had led to these collaterals, similar to those observed in cases with native portal vein thrombosis with or without liver cirrhosis [8–11]. With this in mind, a decompressive procedure similar to those applied in portal hypertension was attempted first. However, serum creatinine started to rise in association with bleeding episodes and severe infection problems. In the end, pancreatectomy and segmental jejunal re-section were necessary to stop the hemorrhage and save the kidney transplant.
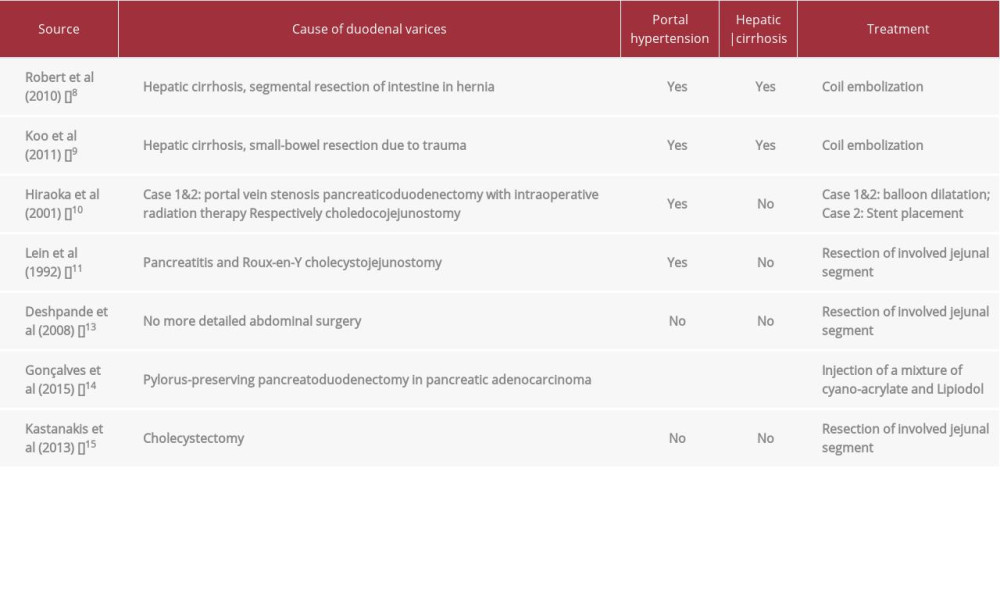
Ectopic varices account for up to 5% of all variceal bleeding episodes [12]. Abdominal surgery seems to be an important risk factor for the development of jejunal varices. With or without hepatic cirrhosis, varices can occur, and the treatment can be tried with angiographic interventions. However, in life-threatening uncontrolled bleeding, resection of the involved jejunal segment may be necessary. Case reports of duodenal varices in the literature (without pancreas-kidney transplant) are summarized in Table 1 [8–11,13–15].
Diagnostic and therapy are challenging, as in our case, and our literature search found the following case reports of jejunal varices in pancreas-kidney-transplanted patients. Rostambeigi et al [16] published a case report with intention of embolization of the jejunal varices in a non-cirrhotic patient.
In the end, segmental jejunal resection was performed. The authors describe the trauma of surgery, peripancreatic graft infection, poor venous drainage, or episodes of rejection as possible reasons for development of jejunal varices. The case report of Fontana et al [7] described embolization as a successful procedure to stop the hemorrhage of jejunal varices during the waiting time for a new liver transplant in a patient with liver cirrhosis.
What also made our case unique from our point of view was the successfully paused immunosuppressive therapy during most of the ICU stay in our hospital. CD4-positive T lymphocytes were monitored and were low over a long period, suggesting immunodeficiency as well as hepatitis E infection and recurrent septic episodes. After successfully stopping the hemorrhage and its complications, CD4 T cell numbers were slowly rising to normal ranges, and immunosuppressive therapy with tacrolimus and prednisolone was restarted. In our case, there were no findings of donor-specific human leukocyte antigen-antibodies in the single-antigen bead assay, suggesting sub-clinical humoral rejection or long-term damages to the renal transplant despite stopping conventional immunosuppressive therapy over about 2 months during this complicated course of disease. We propose that CD4 T cell monitoring could make tailored immunosuppression possible in solid-organ transplant patients with life-threatening complications. Referral of the patient to specialist centers is preferred by Helmy et al [17]. It might be possible to save the pancreas transplant by embolization. In our case, resection was necessary to save the kidney transplant and the patient’s life.
Conclusions
This report is of a rare case of portal hypertension as a long-term complication of transplant surgery. Although acute venous thrombosis at the anastomotic site is a recognized postoperative complication of pancreatic transplant surgery, this rare case highlights the importance of long-term post-transplant follow-up and the role of diagnostic imaging.
Figures
References:
1.. White A, Shaw J, Sutherland D, Pancreas transplantation: Lancet, 2009; 373; 1808-17
2.. Jiang AT, Rowe N, Sener A, Simultaneous pancreas-kidney transplantation: The role in the treatment of type 1 diabetes and end-stage renal disease: Can Urol Assoc J, 2014; 8(3–4); 135-38
3.. Yadav K, Young S, Finger E, Significant arterial complications after pancreas transplantation – a single-center experience and review of literature: Clinical Transplantation, 2017; 31; e13070
4.. Troppmann C, Complications after pancreas transplantation: Curr Opin Organ Transplant, 2010; 15; 112-18
5.. Sollinger H, Odorico J, Becker Y, One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up: Ann Surg, 2009; 250; 618-30
6.. Rayes N, Seehofer D, Kahl A, Long-term outcome of cytomegalovirus infection in simultaneous pancreas–kidney transplant recipients without ganciclovir prophylaxis: Transpl Int, 2007; 20; 974-81
7.. Fontana I, Bertocchi M, Di Domenico S, Percutaneous embolization of periduodenal varix due to portal hypertension in a patient with kidney– pancreas transplantation: A case report: Transplant Proc, 2010; 42; 2162-63
8.. Robert B, Yzet T, Bartoli E, [Embolisation of recurrently bleeding jejunal varices.]: Gastroenterol Clin Biol, 2010; 34; 100-3 [in French]
9.. Koo S, Jeong S, Jang J, Jejunal variceal bleeding successfully treated with percutaneous coil embolization: J Korean Med Sci, 2012; 27; 321-24
10.. Hiraoka K, Kondo S, Ambo Y, Portal venous dilatation and stenting for bleeding jejunal varices: Report of two cases: Surg Today, 2001; 31; 1008-11
11.. Lein B, McCombs P, Bleeding varices of the small bowel as a complication of pancreatitis: Case report and review of the literature: World J Surg, 1992; 16; 1147-49
12.. Kinkhabwala M, Mousavi A, Iyer S, Bleeding ileal varicosity demonstrated by transhepatic portography: Am J Roentgenol, 1977; 129; 514-16
13.. Deshpande A, Sampat P, Bhargavan R, Bleeding isolated jejunal varices without portal hypertension: ANZ J Surg, 2008; 78; 814-15
14.. Gonçalves B, Bastos P, Leão P, Ectopic varices in a pancreatojejunal anastomosis: A rare cause of hemorrhage: Endoscopy, 2015; 47; E269-70
15.. Kastanakis M, Anyfantakis D, Katsougris N, Massive gastrointestinal bleeding due to isolated jejunal varices in a patient without portal hyper-tension: Int J Surg Case Rep, 2013; 4; 439-41
16.. Rostambeigi N, Shrestha P, Dunn T, Recurrent ectopic variceal bleed after pancreas transplantation with no portal hypertension: Case report and outcomes of endovascular onyx embolization: Vasc Endovascular Surg, 2019; 53(5); 415-19
17.. Helmy A, Al Kahtani K, Al Fadda M, Updates in the pathogenesis, diagnosis and management of ectopic varices: Hepatol Int, 2008; 2; 322-34
Figures
In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250