25 May 2022: Articles 
Progressive Worsening of Neurological Manifestations in HIV-Associated Opportunistic Central Nervous System (CNS) Infection Patients After COVID-19 Vaccinations: A Possible Co-Incidence Causality
Unusual clinical course, Rare coexistence of disease or pathology
Faishal Hanif1ABEF, Sekar Satiti1ADE, Subagya Subagya1DE, Heni Retnowulan2DE, Yanri Wijayanti Subronto2DEF, Deshinta Putri Mulya2DEF, Mawaddah Ar Rochmah1ADEF*DOI: 10.12659/AJCR.936257
Am J Case Rep 2022; 23:e936257
Abstract
BACKGROUND: The iceberg phenomenon (in which the most of a problem is invisible) of people living with HIV/AIDS, particularly those with unknown HIV status, has been epidemiologically challenging. Central nervous system (CNS) opportunistic infections in patients with HIV/AIDS are one of the leading causes of morbidity and mortality in people living with HIV/AIDS. There are currently limited data on the immunogenicity, safety, and efficacy of COVID-19 vaccines in people living with HIV/AIDS with its associated opportunistic CNS infections as well as those without antiretroviral treatment.
CASE REPORT: Two young men with previously unknown HIV status and its related opportunistic infections received their first doses of COVID-19 vaccine (Vero Cell), inactivated. Both patients had the risk factor of having sex with men (men who have sex with men). Fever and first neurological symptoms occurred within the first few days after vaccination. Both patients were hospitalized and were tested positive for HIV for the first time. Both were further diagnosed from brain imaging as having CNS opportunistic infections. A presumptive diagnosis of cerebral toxoplasmosis was established as the working diagnosis according to the laboratory and epidemiological factors. Despite the treatment, neurological and clinical deficits worsened and eventually led to death in both patients.
CONCLUSIONS: The causality analyses showed that both adverse events had a possible inconsistent causal relationship to COVID-19 vaccination. Our cases may reflect the need for further studies on the safety of COVID-19 vaccines in people with HIV/AIDS-associated CNS opportunistic infection as well as people with HIV/AIDS who never receive antiretroviral treatment (ART).
Keywords: COVID-19 Vaccines, HIV, Toxoplasmosis, Cerebral, AIDS-Related Opportunistic Infections, Acquired Immunodeficiency Syndrome, Brain, COVID-19, Central Nervous System, HIV Infections, Homosexuality, Male, Humans, Incidence, Male, Sexual and Gender Minorities, Vaccination
Background
CNS opportunistic infections in patients with HIV/AIDS are one of the leading causes of morbidity and mortality in PLWHA, despite the introduction to ART [1–3]. Some agents causing central nervous system (CNS) opportunistic infections, such as
The COVID-19 pandemic has been declared since March 2020 by the WHO until recently. Development and distribution of vaccines against SARS-COV-2 have been accelerated to end the pandemic era to protect healthcare systems, restore the global economy, and reinstate normal social interactions. PLWHA, at any stage of the disease, are recommended by the WHO and CDC as priority targeted subjects for COVID-19 vaccination [4]. The data on efficacy and safety of COVID-19 vaccine in PLWHA are still limited to those with stable HIV stage who have been receiving ART. Two studies reported that the COVID-19 vaccine ChAdOx1 nCoV-19 was safe and immunogenic in PLWHA with ART and CD4+ count >350 cells/µl [5,6]. Other studies reported that inactivated COVID-19 vaccine showed safety in PLWHA who received ART but a poor immunological response was observed in those with CD4+ count <350 cells/µl [7]. Furthermore, there are also no data reporting that these COVID-19 vaccines are inefficacious and unsafe or interfere with ART [5–8]. Here, we report 2 cases of young men with previously unknown HIV status whose neurological symptoms emerged and worsened progressively after receiving the first dose of vaccine against SARS-CoV-2.
Case Reports
PATIENT 1:
A 23-year-old single man was brought to the ED with acute onset of high fever and weakness in his left arm and leg. The fever had persisted for the last 10 days despite taking over-the-counter paracetamol. He felt weakness in his left leg and arm for the last 5 days. He reported that he felt low-intensity headaches once or twice a week in the last few months. The headache was relieved with rest and over-the-counter paracetamol.
The patient received the first dose of COVID-19 vaccine (Vero Cell, inactivated) 2 days before the fever started. A marked body weight reduction and history of having sex with men (men who have sex with men – MSM) were reported. He said he had been healthy prior to the present illness.
The patient presented with general weakness, body temperature of 38.5°C, and fully conscious. On neurologic examination, upper motor neuron type of left facial nerve weakness was found. His left arm and leg could only move against gravity, without any activity against the examiner’s low resistance. Increased physiological reflexes, pathological reflexes, and clonus were found in his left extremities. Neck stiffness and Kernig sign were also found.
On laboratory examination, normal white blood cell (WBC) count with low lymphocyte count and low presumptive CD4+ count of 154 were found (Table 1). Critical serum hyponatremia was also found. He tested reactive and positive for HIV-rapid and ELISA HIV, respectively. TORCH examination showed increased serum IgG levels of anti-toxoplasma, anti-CMV, and anti-rubella, with high avidity on both anti-toxoplasma and anti-CMV IgG. A chest X-ray showed suspected signs of atypical pneumonia in his right lung (Figure 1A). He was scheduled for brain imaging and lumbar puncture, but he refused the lumbar puncture procedure.
He was presumptively diagnosed with cerebral toxoplasmosis and was treated with oral pyrimethamine. Intravenous dexamethasone and CNS-dosing ceftriaxone were given as empirical therapy for possible bacterial meningoencephalitis. Intravenous paracetamol was given as an antipyretic for fever. His serum sodium level was corrected with intravenous hypertonic saline. On the third day of inpatient care, the patient decided to be discharged from the hospital.
Three days after hospital discharge, the patient was brought again to the ED due to seizures and decreased consciousness. His family witnessed general tonic clonic seizure 3 times, with duration of 2–3 min each in the last 12 h. His fever, left extremity weakness, and headache persisted.
On this second admission, he had body temperature of 39.2°C, GCS of E3V2M5, heart rate 111 beats per minute, and respiration rate 22 times per minute. The left facial nerve weakness, left extremity weakness, neck stiffness, Kernig sign, increased physiological reflexes, pathological reflexes, and clonus findings were consistent with his first admission.
On laboratory examination, a normal WBC with low lymphocyte count and low presumptive CD4+ count of 174, serum hyponatremia, and hypercoagulation were found. Urinalysis showed marked bacteriuria and leukocyturia but the history of urinary tract symptoms was unknown. His chest X-ray showed right para-hilar infiltrates, suggesting pneumonia from tuber-culous infection (Figure 1B). A contrast head CT scan showed multiple foci in bilateral frontal and parietal lobes with perifocal edema with midline shift to the left (Figure 2). According to these examinations, our working diagnosis for this patient was cerebral toxoplasmosis with a differential diagnosis of tuberculous meningoencephalitis, as well as pulmonal tuberculosis in an HIV/AIDS patient. The chronological timeline of the patient’s disease course is presented in Figure 3.
The presumptive cerebral toxoplasmosis was treated with oral pyrimethamine and cotrimoxazole. Intravenous dexamethasone and Ceftazidime were given as empirical therapy for possible bacterial meningoencephalitis. A fixed-dose antituberculosis drug combination containing rifampicin, isoniazid, pyrazin-amide, and ethambutol was given for the pulmonary tuberculosis as well as the possible tuberculous meningoencephalitis.
The seizure was treated with intravenous phenytoin and intravenous paracetamol was given as an antipyretic for fever. Serum hyponatremia was corrected using hypertonic saline. On daily evaluation, his consciousness improved to somnolence and his general tonic seizure did not re-occur. However, continuous focal myoclonus of the right arm was observed. Intravenous phenytoin was then switched into oral valproic acid. His focal myoclonus stopped 1 day following the first administration of valproic acid. Unfortunately, his condition worsened on the third day of his second hospitalization. Sepsis was confirmed from his clinical and laboratory findings. He eventually developed acute respiratory distress syndrome and septic shock, which led to death.
PATIENT 2:
A 23-year-old single man was brought to the ED with altered mental status preceded by acute exacerbating headache and fever. His family stated that he was just discharged from another hospital 3 days before his current admission, due to fever, headache, and 2 separate events of general tonic clonic seizure. Non-contrast head CT scan from his first inpatient care showed no abnormality. In addition, he tested reactive on the HIV-rapid test. He had been free from fever, headache, and seizure after his first inpatient care in the previous hospital before the symptoms occurred again and worsened 1 day before his current admission.
His family said that he had a history of MSM and reported significant weight loss. He was also reported to have no symptoms of any disease as far as his family recalled. The patient received his first dose of COVID-19 vaccine (Vero Cell, inactivated) 14 days before current hospital admission.
On his current admission, he had GCS of E4V4M5 and body temperature of 38.6°C with normal blood pressure, respiration rate, and heart rate. A neurological examination showed neck stiffness and Kernig sign with normal cranial nerve examinations and no lateralization. The motoric examination, physiological reflexes, and pathological reflexes were normal. Laboratory examination showed normal WBC with normal lymphocyte count and estimated CD4+ count of 382. Serum hypercoagulation was found, with D-dimer count of 1785 µg/ml. He tested positive for ELISA HIV, TPHA, and VDRL. TORCH examination showed elevated serum IgG levels of anti-toxo-plasma, anti-CMV, and anti-rubella, with high avidity on both anti-toxoplasma and anti-CMV IgG (Table 1). A blood culture grew no bacteria or fungi.
Lumbar puncture was performed on the day after his admission, and his CSF analysis showed pleocytosis (127 mononuclear cells and 3 polymorphonuclear cells/µl), elevated protein (0.14 gr/dl), and decreased CSF/serum glucose ratio (38%). Immunochromatographic testing for Cryptococcus and rapid molecular test for tuberculosis showed negative results.
A chest X-ray showed para-hilar and para-cardiac infiltrates in his left lung (Figure 4). A contrast brain MRI showed multiple lesions in both cerebral and cerebellar hemispheres, suggesting cerebral toxoplasmosis (Figure 5). He was diagnosed with presumptive cerebral toxoplasmosis and atypical community-acquired pneumonia in an HIV/AIDS patient. The chronological timeline of the patient’s disease course is shown in Figure 3.
He was given oral pyrimethamine to treat the presumptive cerebral toxoplasmosis. Intravenous dexamethasone and CNS-dosing ceftriaxone were given as empirical therapy for possible bacterial meningoencephalitis. The atypical community-acquired pneumonia was treated with oral azithromycin. Oral phenytoin and paracetamol were given as antiepileptic and antipyretic drugs, respectively. On the fifth day of inpatient care, he developed progressive decrease of consciousness, worsening headache, frequent vomiting, and seizures, suggesting elevated intracranial pressure, which led to death.
Discussion
Epidemiologically, our national and global society has been facing the iceberg phenomenon of PLWHA, particularly those whose HIV status is not known, as in 2 patients reported here [9,10]. There were 37.6 million PLWHA across the globe in 2020, and 95.5% of them were adults. In the same year, the global HIV incidence was 1.5 million individuals [9]. This estimated HIV incidence number includes individuals who might have acquired HIV infection without knowing it. In Indonesia, the estimated number of PLWHAs in 2020 was 543 100, with 32 293 new cases, and a total of 409 857 people living with HIV/AIDS were reported in the annual HIV/AIDS report from the Ministry of Health of the Republic of Indonesia [10]. HIV status may be crucial in the current COVID-19 era due to the growing evidence that suggest PLWHA as an independent risk factor for higher morbidity and mortality in COVID-19 outcomes [4,11]. The increased risk of morbidity and mortality due to COVID-19 in PLWHA was also reported despite the successful HIV treatment, suggesting immune deficiency as the culprit [12]. Therefore, there are national and international consensuses regarding the necessity of COVID-19 vaccinations in PLWHA, despite the limited data on the efficacy and safety of the vaccines in these subpopulations [13]. In some countries, including Indonesia, it is necessary for people with certain medical conditions, including PLWHA whose HIV infection status has been known, to have a physician’s recommendation for COVID-19 vaccination in spite of the safety claim from the WHO and CDC for COVID-19 vaccinations in PLWHA [4,14].
We performed causality analyses on the adverse events following immunization in these 2 patients (Supplementary Material). The results showed that both adverse events had an inconsistent causal relationship with immunization [15]. Therefore, these cases were both coincidental events following COVID-19 vaccinations in patients with unknown medical conditions that turned out to be PLWHA with CNS opportunistic infections. The occurrence of CNS opportunistic infections was not only caused by the noncompliance with ART and ART’s resistance in HIV patients with ART, but also the unawareness of the HIV-positive status in some individuals [2]. Furthermore, these HIV-related CNS opportunistic infections can cause major morbidity and mortality in HIV patients despite the ART, since these opportunistic infections may reflect the low CD4+ count as well as the severe degree of HIV infections, particularly in low-to-middle income countries [2,16]. Diagnosis evaluation in HIV-related CNS opportunistic infections should be based on local neuroepidemiology, the degree of immunosuppression, and individual clinical, laboratory, and brain imaging features throughout the disease course [16].
There are currently limited data on the immunogenicity of COVID-19 vaccines in PLWHA, although evidence shows the importance of CD4+ and CD8+ T cells in the immunologic memory component in the host response to SARS-COV-2 infection [17,18]. Previous studies suggested that immune response in recombinant hepatitis B vaccination in PLWHA whose CD4+ lymphocytes are <50/mm3 was not effective [19]. Furthermore, shorter duration of seroprotection in PLWHA was confirmed after vaccinations against hepatitis B, hepatitis A, measles, mumps, and rubella, tetanus, poliomyelitis, and pertussis, and some other pathogens [20]. Immunization while PLWHA are on ART, as well as increasing the antigen dose, are known as 2 strategies for improving the long-term seroprotection in vacci-nation in this subpopulation [20]. Unfortunately, neither strategy was applied in our patients due to their unknown HIV status.
Our current knowledge on COVID-19 vaccination in PLWHA was based on the findings from trials involving stable HIV patients that suggest the safety, tolerability, and immunogenicity of COVID-19 vaccines [5–8]. This is also emphasized by the recommendations of the WHO and CDC for COVID-19 vacci-nation in PLWHA [4]. To date, there is no clear understanding of adverse events following COVID-19 vaccination in PLWHA with previously unknown HIV status. The closest study we found to our current report was a case report in a treatment-naïve HIV-positive patient who experienced deterioration of HIV infection after receiving the first dose of an inactivated COVID-19 vaccine [21]. This report suggested the possibility of activated HIV infection that directly increased the rate of CD4+ T cells destruction, thus causing serial depletion of CD4+ T cells. This report also suggested that ART initiation in the case of treatment-naïve PLWHA could be beneficial to preserve immune function, prevent secondary HIV transmission, and prevent complications of health conditions or procedures, including vaccines [21]. In addition, infection with HIV-1 and SARS-CoV-2 causes marked CD4+ T cell lymphopenia by the mechanisms of direct CD4+ T cells attacks, immune activation, and CD4+ T cell redistributions [22]. As for COVID-19 vaccination, proper CD4+ T cell function is necessary for an effective vaccine response [22].
In our present cases, both patients were diagnosed with CNS opportunistic infections at the same time as their confirmed HIV infection. At this point, the CNS opportunistic infections in these patients may have reflected the advanced HIV infection [1], despite the presumptive CD4+ count in our reported cases. In other words, the presumptive cerebral toxoplasmosis found in both patients was an AIDS-defining illness regardless of CD4+ count. Based on the previously reported case, we speculate that possible activation of HIV infection after inactivated COVID-19 vaccine injection led to increased CD4+ T cells destruction, which in turn caused progressive clinical deteriorations in the reported cases [21]. However, it should be taken into account that this speculation is based on only a single case report of a treatment-naïve HIV-positive patient and the findings cannot be generalized to other patients with HIV. It is important to follow the COVID-19 vaccination guidelines according to the regional or national regulations, particularly for those with comorbidities. The Indonesian Society of Internal Medicine recommended that COVID-19 vaccination for PLWHA can be performed if their condition is stable and they are receiving routine ART [23].
There are limitations of our study: (i) CNS opportunistic infections were established according to the presumptive diagnosis criteria of cerebral toxoplasmosis and its differential diagnosis, (ii) CD4+ count was performed using presumptive CD4 count, and whether it reflects the true CD4+ count is unclear, and (iii) there is limited evidence of vaccination error. There is a need for further studies on the safety of COVID-19 vaccines in PLWHA without ART as well as HIV/AIDS-associated CNS opportunistic infections. The early initiation of ART after COVID-19 vaccination was previously reported to be beneficial in treatment-naïve PLWHA, but this can only be performed if the HIV status is determined.
Conclusions
Here, we presented 2 cases that were possible coincidental events between treatment-naïve PLWHA with CNS opportunistic infections after COVID-19 vaccinations. Our cases may extend the needs for further studies on the safety of COVID-19 vaccines in HIV/AIDS-associated CNS opportunistic infection as well as HIV/AIDS patients who never received antiretroviral treatment (ART).
Tables
. Patient 1Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesVaccine type: inactivated COVID-19 vaccineStep 2. Questions of Adverse Events Following Immunization.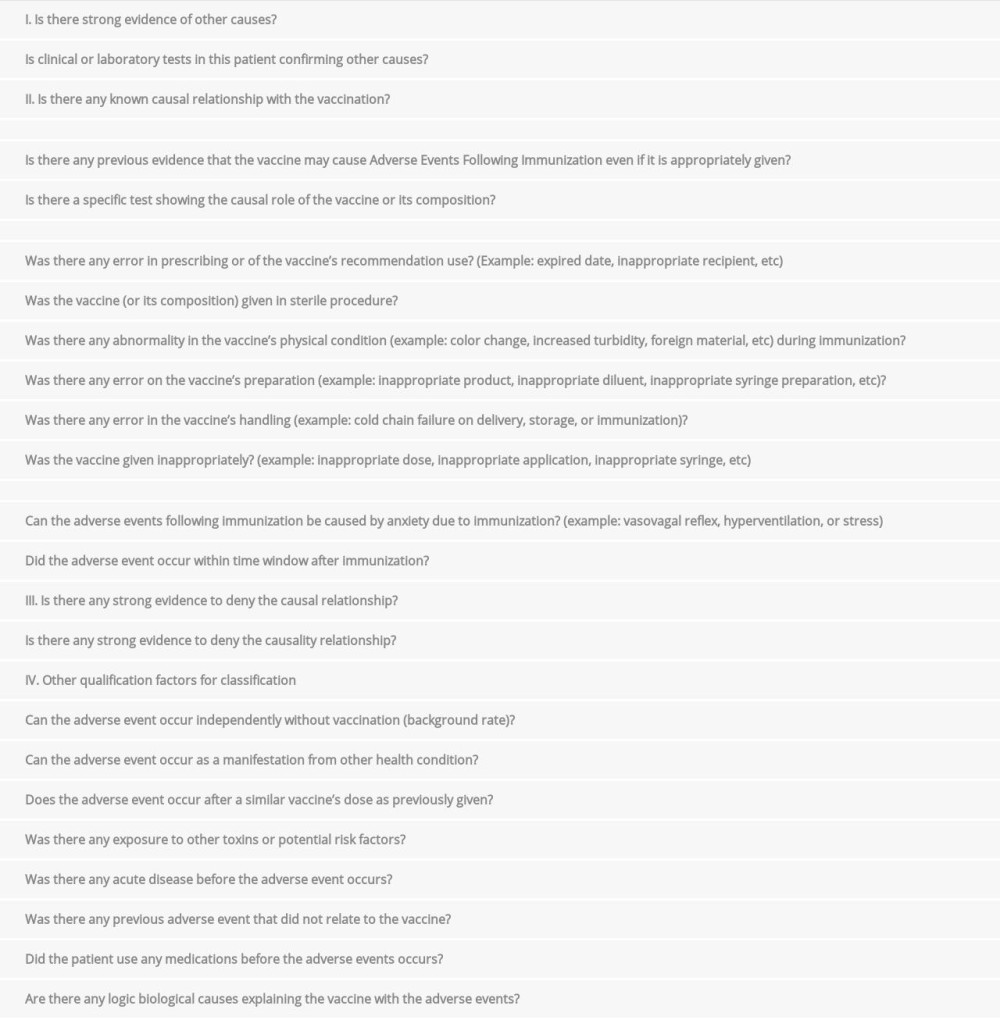 . Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization.
. Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization.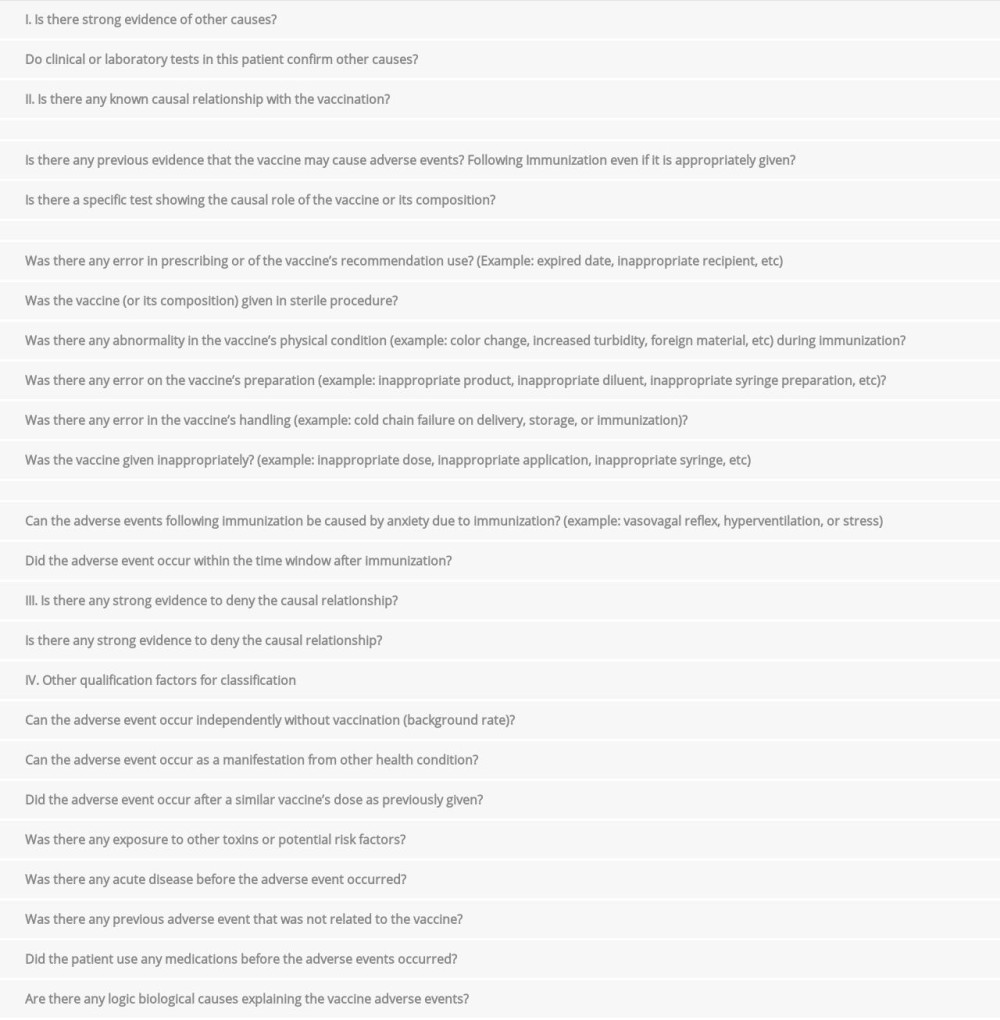
References:
1.. Tan IL, Smith BR, von Geldern G, HIV-associated opportunistic infections of the CNS: Lancet Neurol, 2012; 11; 605-17
2.. Bowen LN, Smith B, Reich D, HIV-associated opportunistic CNS infections: Pathophysiology, diagnosis, and treatment: Nat Rev Neurol, 2016; 12; 662-74
3.. Thakur KT, CNS infections in HIV: Curr Opin Infect Dis, 2020; 33; 267-72
4.. : Coronavirus disease (COVID-19): HIV antiretrovirals [Internet], WHO [cited 2021 January 7]. Available from: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-hiv-and-antiretrovirals
5.. Frater J, Ewer KJ, Ogbe A, Safety and immunogenicity of the ChAdOx1 nCov-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial: Lancet HIV, 2021; 8(8); e474-85
6.. Madhi SA, Koen AL, Izu A, Safety and immunogenicity of the ChAdOx1 nCov-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: An interim analysis of a randomize, double-blind, placebo-controlled, phase 1B/2A trial: Lancet HIV, 2021; 8(9); e568-80
7.. Liu Y, Han J, Li X, COVID-19 vaccination in people living with HIV (PLWH) in China: A cross sectional study of vaccine hesitancy, safety, and immunogenicity: Vaccines, 2021; 9; 1458
8.. Wu S, Zhang Y, Ming F, Adverse events of inactivated COVID-19 vaccine in HIV-infected adults: AIDS Res Ther, 2021; 18; 92
9.. : The Global HIV/AIDS epidemic [Internet], US Department of Health and Human Services [cited2021 January 7]. Available from:https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics
10.. : Estimation and Projection of HIV/AIDS in Indonesia years 2005–2020, 2017, Jakarta, Ministry of Health of Republic Indonesia
11.. Geretti AM, Stockdale AJ, Kelly SH, Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): A prospective observational study: Clin Infect Dis, 2021; 73; e2095
12.. Hoffmann C, Casado JL, Härter G, Immune deficiency is a risk factor for severe COVID-19 in people living with HIV: HIV Med, 2021; 22; 372-78
13.. Jilich D, Skrzat-Klapaczyńska A, Fleischhans L, National strategies for vaccination against COVID-19 in people living with HIV in Central and Eastern European region: HIV Med, 2022; 23; 546-52
14.. : COVID-19 and HIV [Internet], Centers for Disease Control and Prevention [cited 2021 January 7]. Available from: https://www.cdc.gov/hiv/basics/covid-19.html
15.. : Module 3 Adverse events following immunization [Internet], WHO [cited 2021 January 7]. Available from: https://www.who.int/vaccine_safety/initiative/tech_support/Part-3.pdf?ua=1
16.. Vidal JE, HIV-related cerebral toxoplasmosis revisited: current concepts and controversies aof an old disease: J Int Assoc Provid AIDS Care, 2019; 18; 1-20
17.. Plummer MM, Pavia CS, COVID-19 vaccines for HIV-infected patients: Viruses, 2021; 13; 1890
18.. Chen Z, Wherry EJ, T cell responses in patients with COVID-19: Nat Rev Immunol, 2020; 20; 529-35
19.. Pasricha N, Datta U, Chawla Y, Immune responses in patients with HIV infection after vaccination with recombinant Hepatitis B virus vaccine: BMC Infect Dis, 2006; 6; 65
20.. Kernéis S, Launay O, Turbelin C, Long-term immune responses to vaccination in HIV-infected patients: A systematic review and meta-analysis: Clin Infect Dis, 2014; 58; 1130-39
21.. Gong C, Song X, Li X, Immunological changes after COVID-19 vaccination in an HIV-positive patient: Int J Infect Dis, 2021; 117; 230-32
22.. Peng X, Ouyang J, Isnard S, Sharing CD4+ T cell loss: When COVID-19 and HIV collide on immune system: Front Immunol, 2020; 11; 596631
23.. : PAPDI Recommendation on COVID-19 vaccination in People with Comorbidities [internet], Perhimpunan Dokter Spesialis Penyakit Dalam Indonesia (Indonesian Society of Internal Medicine) [cited 2022 January 21]. Available from: https://www.papdi.or.id/berita/info-papdi/1024-rekomendasi-papdi-tentang-pemberian-vaksinasi-covid-19-pada-pasien-dengan-penyakit-penyerta-komorbid-revisi-18-maret-2021
Figures
Tables
 . Patient 1Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesVaccine type: inactivated COVID-19 vaccineStep 2. Questions of Adverse Events Following Immunization.
. Patient 1Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesVaccine type: inactivated COVID-19 vaccineStep 2. Questions of Adverse Events Following Immunization. . Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization.
. Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization. . Patient 1Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesVaccine type: inactivated COVID-19 vaccineStep 2. Questions of Adverse Events Following Immunization.
. Patient 1Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesVaccine type: inactivated COVID-19 vaccineStep 2. Questions of Adverse Events Following Immunization. . Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization.
. Patient 2Step 1. Background.Data availability: YesDoes the diagnosis meet the case definition? YesStep 2. Questions of Adverse Events Following Immunization. In Press
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942864
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








