13 October 2022: Articles 
Columnar Metaplasia of the Esophagus Presenting as Iron Deficiency Anemia in Children with Neurologic Impairment or Congenital Esophageal Atresia
Challenging differential diagnosis, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Melissa R. Van Arsdall1ABCDEF*, Supriya Nair1BDEF, Lindsay M. Moye2BDEF, Trinh T. Nguyen3BDEF, Zeina M. Saleh1DEF, J. Marc RhoadsDOI: 10.12659/AJCR.937255
Am J Case Rep 2022; 23:e937255
Abstract
BACKGROUND: Columnar metaplasia of the lower esophagus includes both gastric and intestinal metaplasia. Children with severe neurologic impairment and congenital esophageal atresia often have gastroesophageal reflux disease, which can lead to Barrett’s esophagus, a form of lower esophageal columnar metaplasia and precursor to esophageal adenocarcinoma, with some, but not all, guidelines specifically requiring the presence of intestinal metaplasia for diagnosis. This case series illustrates how iron deficiency anemia may be the primary symptom of esophageal columnar metaplasia in such children and how upper endoscopy is essential in their initial and ongoing evaluation.
CASE REPORT: We review 5 cases of columnar metaplasia of the lower esophagus in children, 3 with severe neurologic impairment and 2 with esophageal atresia. Each child presented with marked iron deficiency anemia and minimal-to-no gastrointestinal symptoms.
CONCLUSIONS: We conclude that columnar metaplasia of the esophagus may present with iron deficiency anemia in children with neurologic impairment or congenital esophageal atresia, even if without overt gastrointestinal symptoms. Accordingly, we propose that early endoscopic evaluation should be considered in this specific patient population. Based on our literature review, we also emphasize the need for guidelines on the endoscopic surveillance of such children with any type of columnar metaplasia of the lower esophagus, given the associated risk of malignant transformation.
Keywords: Anemia, Iron-Deficiency, Barrett Esophagus, esophageal atresia, Metaplasia, neurodevelopmental disorders, Pediatrics, Anemia, Child, Esophageal Neoplasms, Humans, Iron Deficiencies, Nervous System Diseases
Background
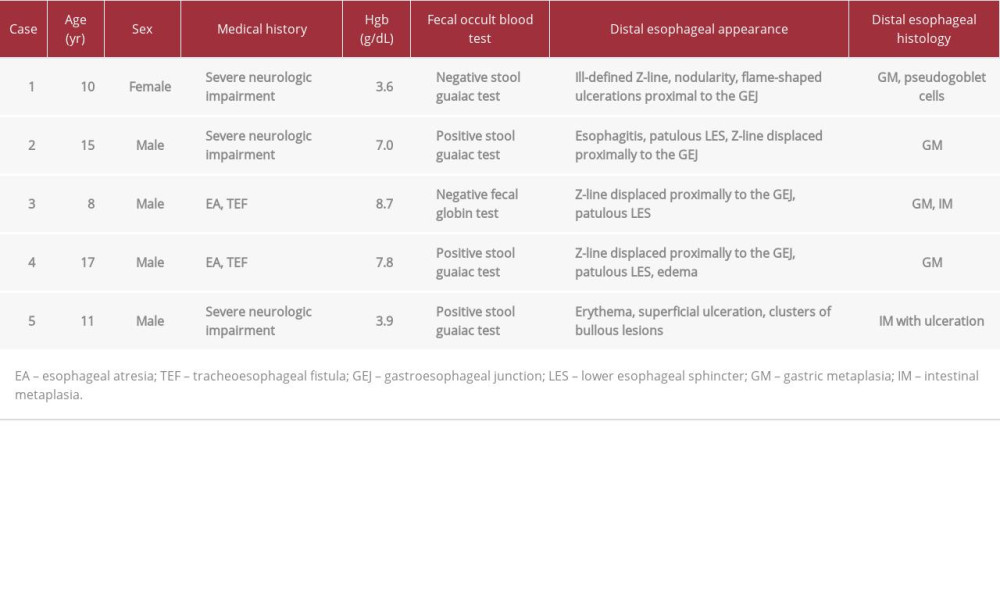
Barrett’s esophagus (BE) is a precursor to esophageal adeno-carcinoma and is a consequence of gastroesophageal reflux disease (GERD) [1]. BE is rare in children, with BE found in <0.25% of 6731 children undergoing upper endoscopic evaluation in one study [2]. However, BE has been well-documented, both in children with neurologic impairment and in those with a history of esophageal atresia (EA), as they are particularly prone to developing GERD [3–7]. Various factors limiting detection of BE and differing diagnostic criteria for BE have important implications for subsequent endoscopic surveillance [1,8–20]. BE is composed of columnar metaplasia (CM) in the lower esophagus, and CM is present in both gastric metaplasia (GM) and intestinal metaplasia (IM). Goblet cells are seen in IM, and these are required for the diagnosis of BE according to some, but not all, guidelines [1,15–19]. While gastrointestinal bleeding and iron deficiency anemia (IDA) have been found in up to one-third of patients with columnar cell-lined esophagus in some studies, evaluation for GERD-related esophageal damage is not part of the routine evaluation of anemia in children [21–23]. In this series, we discuss 5 children, either with neurologic impairment or a history of EA, who presented with IDA but no gastrointestinal (GI) symptoms and who were ultimately found to have CM of the lower esophagus (Table 1).
Case Reports
CASE 1:
An 8-year-old girl was referred to the gastroenterology service for refractory IDA of unclear etiology. She had a history of extreme prematurity, neonatal intraventricular hemorrhage, ventriculoperitoneal shunt placement for hydrocephalus, agenesis of the corpus callosum, profound neurodevelopmental delay, blindness, seizure disorder, cerebral palsy, and cranioplasty for multiple craniofacial abnormalities. Her diet consisted of pureed foods and copious amounts of cow’s milk orally. She rarely vomited, but she exhibited posturing and often slapped herself.
Upon initial hematology evaluation, her hemoglobin (Hgb) was very low (4.4 g/dL; reference range 11.5–15.5 g/dL), and her ferritin was low (2 ng/mL; reference range 5–204 ng/mL). Restriction of cow’s milk intake was advised, and oral iron therapy was started. Six months thereafter, her Hgb improved to 13 g/dL, and her ferritin normalized. At follow-up 5 months later, she was no longer on supplemental iron, and she continued to be maintained on a well-balanced diet with adequate iron content and with avoidance of cow milk. With no overt signs of blood loss, her Hgb was again low (7.1 g/dL), reticulocyte count elevated (2.6%; reference range 0.5–1.5%), ferritin low (1 ng/mL), mean corpuscular volume (MCV) low (54.4 fL; reference range 80–98 fL), and platelet count normal (358×103/microL; reference range 133–450×103/microL). She had a negative a Coombs test, normal renal function, and a negative fecal occult blood test. She was restarted on oral iron and subsequently referred to gastroenterology.
Further evaluation showed a normal gastrin level and negative tissue transglutaminase (tTG) immunoglobulin A (IgA) antibody screening for celiac disease. Fecal calprotectin was 199 µg/g (normal <162.9 µg/g), suggesting mild GI inflammation. Upper GI endoscopy showed esophagitis, a 3-cm sliding hiatal hernia, and flame-shaped projections just above the gastroesophageal junction (GEJ). Rapid urease testing was negative for
At age 9.5 years, she underwent Nissen fundoplication with gastrostomy tube placement. Postoperatively, she remained on iron and PPI treatment, with improved anemia (Hgb 13.5 g/dL, MCV 82.2 fL), but relatively low iron stores (ferritin 11 ng/mL). Continued iron therapy was advised.
After being lost to follow-up for a year, and having been off of PPI treatment and supplemental iron, she was admitted to the hospital with Hgb of 3.6 g/dL, MCV 55.7 fL, reticulocyte count of 4.3%, and normal platelet count at 316×103/microL, with no obvious reflux symptoms. Her diet continued to contain the recommended daily intake of iron. Repeat endoscopy then revealed an irregular, ill-defined Z-line, nodularity in the distal esophagus, and flame-shaped projections above the GEJ, accentuated with blue light (Figure 1A, 1F). Esophageal biopsies showed gastric epithelium (GM) with reactive changes and pseudogoblet cells, mimicking the appearance of goblet cells (Figure 1K). Repeat rapid urease testing and stains for fungus, H. pylori, CMV, and HSV 1 and 2 were all negative. Colonoscopy biopsies, capsule enteroscopy, Meckel’s scan, and a pH-impedance study were all normal.
CASE 2:
A 15-year-old boy with congenital Dandy-Walker malformation, chromosome 3 deletion, seizure disorder, marked global developmental delay, and scoliosis developed weight loss of 6.8 kg, about 25% of his body weight, over 2 years, despite high-calorie tube feedings with a cow’s milk-free and gluten-free food blend with adequate iron content. He had a history of GERD, with ulcerative esophagitis 3 years earlier, and he was maintained on PPI therapy, with no vomiting. His body mass index was well below the fifth percentile, and physical exam showed severe scoliosis, hypertonia, microphthalmia, partial deafness, and self-stimulatory behavior. Labs showed Hgb 7.0 g/dL, red blood cell distribution width (RDW) of 18% (reference range 11.5–14.5%), MCV 62 fL, Mentzer index 15.6 (reference >13 in IDA), platelets 366×103/microL, negative tTG IgA antibody, hemoccult positive stool, and stool studies negative for infection. There were no signs of bleeding outside the GI tract.
Endoscopy revealed active distal esophagitis with large flame-shaped projections of erythematous mucosa about 4 cm above the gastroesophageal junction (GEJ) and a patulous-appearing lower esophageal sphincter (LES) (Figure 1B, 1G). The stomach appeared normal, while the duodenum appeared heterogeneous and irregular.
Biopsies showed GM (Figure 1L) and junctional type columnar mucosa, along with pseudogoblet cells and scattered neutrophils in the lower esophagus below the transition of the flame-like mucosa, with mild reflux esophagitis above the Z-line. Stomach biopsies were unremarkable and negative for H. pylori organisms. The duodenum showed villus atrophy without intraepithelial lymphocytes or eosinophils, presumably secondary to malnutrition.
He was admitted to the hospital, placed on supplemental iron and amino acid formula, increased PPI dosing, and pancreatic enzymes. He gained weight rapidly without evidence of refeeding syndrome and was subsequently discharged. Follow-up CBC 1 year thereafter was normal, with Hgb 13.9 g/dL, MCV 92.8 fL, RDW 13%, and platelets 191×103/microL.
CASE 3:
An 8-year-old boy with a history of EA and tracheoesophageal fistula (TEF), repaired at birth and later complicated by esophageal stricture requiring multiple dilations, presented from another institution with anemia. An upper GI series at 7 years of age showed mild mid-segment narrowing of the esophagus, without obstruction. He had been maintained on PPI therapy since infancy. He had no dysphagia, vomiting, melena, abdominal pain, anorexia, weight loss, hemoptysis, or hematuria.
Physical exam was normal. Labs showed IDA with Hgb 8.7 g/dL, MCV 58.5 fL, RDW 18.5%, iron 16 µg/dL (reference range 45–160 µg/dL), iron saturation 3% (reference range 12–57%), and ferritin 2 ng/mL. Platelets (383 103/microL), white blood cell count, and comprehensive metabolic profile were normal. Fecal globin was negative. Upper endoscopy revealed narrowing of the middle to distal esophagus, displacement of the Z-line proximal to the GEJ, and a patulous LES (Figure 1C, 1H). The stomach and duodenum appeared normal.
Histology of the distal esophagus showed GM with chronic gastritis and focal IM without dysplasia, and Alcian blue stain highlighted goblet cells in the distal esophagus, consistent with BE (Figure 1M). Mid-esophageal biopsies showed reflux esophagitis, while proximal esophageal, stomach, and duodenal biopsies were normal.
He has since been referred to surgery for Nissen fundoplication. He is currently on high-dose PPI therapy and supplemental iron, with his most recent Hgb at 11.2 g/dL, iron 33 µg/dL, and ferritin 3 ng/mL.
CASE 4:
A 17-year-old male, born with EA and TEF, was referred by his hematologist for IDA. He had undergone surgical repair of his EA and TEF, along with Nissen fundoplication, during early infancy. He had been on omeprazole sporadically for most of his life, except for the preceding 3 years. He had initially presented to his pediatrician with progressively worsening shortness of breath and decreased soccer performance over a 6-month period; he denied any other symptoms. His Hgb was then 7.3 g/dL, with MCV at 60.1 fL. Subsequent evaluation by hematology showed Hgb 7.8 g/dL, MCV 59 fL, RDW 19.7%, reticulocyte count 1.5%, iron 22 µg/dL, and ferritin 2 ng/mL. His white blood cell and platelet counts were normal. He was started on supplemental iron. Later evaluation by gastroenterology showed improvement in his Hgb (13.2 g/dL), along with a normal ESR, CRP, total IgA, and negative tTG IgA. His stool was negative for
He returned to the clinic 3 months later and had stopped supplemental iron during the prior 2 weeks due to an upset stomach; he otherwise felt well. His Hgb had declined to 11.0 g/dL, while his iron level was 22 µg/dL. His stool remained positive for occult blood. He had no hematuria and no hemoptysis.
Subsequent endoscopic evaluation revealed mild narrowing in the mid-esophagus, edema in the lower esophagus, displacement of the Z-line proximal to the GEJ, and a patulous LES (Figure 1D, 1I). The previous Nissen fundoplication was not intact. His stomach, duodenum, colon, and rectum appeared normal.
Histology showed reflux esophagitis throughout the esophagus. Biopsies corresponding to the tissue within the “flame” and proximal to the GEJ showed inflamed columnar epithelium, consistent with GM, along with pseudogoblet cells (Figure 1N). Biopsies of the stomach and duodenum were normal. The colon and rectum exhibited eosinophilia but no diagnostic features of inflammatory bowel disease or eosinophilic colitis. He was placed on high-dose PPI, restarted on iron, and referred to surgery for fundoplication revision. One year later, his hemoglobin (13.7 g/dL) and MCV (84.8 fL) were normal.
CASE 5:
An 11-year-old male, with a history of very early preterm birth, intraventricular hemorrhage, cerebral palsy, recurrent pneumonias, GERD, and gastrostomy tube feeding dependence, presented to his pediatrician with pallor. He had had no feeding intolerance, melena, hematochezia, easy bruising, nor vomiting while on long-term lansoprazole for GERD. He had neither hemoptysis nor hematuria. CBC showed a markedly low Hgb of 3.9 g/dL, and he was referred to the hospital for further evaluation.
Physical exam in the emergency room revealed pallor, tachycardia, a systolic ejection murmur, and hemoccult positive stool. Labs then showed Hgb 3.9 g/dL, MCV 58.6 fL, reticulocyte count 6.6%, and RDW 21.1%. Platelets (383×103/microL), white blood cell count, and coagulation profile were normal. A peripheral blood smear was consistent with IDA. He was transfused with packed red blood cells, placed on PPI and octreotide infusions, and admitted to the intensive care unit.
Endoscopic evaluation showed diffuse erythema throughout the esophagus, most pronounced in the distal esophagus. Small, superficial ulcerations and clusters of bullous lesions were also seen in the lower esophagus (Figure 1E, 1J). The stomach and duodenum appeared normal.
Biopsies from the distal esophageal lesions showed fragments of gastric mucosa with goblet cells, consistent with IM, along with mucosal acute and chronic inflammation, and fibrinopurulent exudate, consistent with ulcer (Figure 1O). Stains for CMV, HSV 1 and 2, and fungus were negative. Stomach and duodenal biopsies were normal.
He was placed on supplemental iron and PPI therapy and soon thereafter underwent Nissen fundoplication. Since his surgery, he is no longer anemic (Hgb 15.6 g/dL, MCV 84.4 fL) while off of iron supplementation and while maintained on PPI therapy.
Discussion
The normal esophagus is lined by stratified squamous epithelium. Any type of columnar epithelium found in the esophagus, proximal to the gastroesophageal junction (GEJ), is considered metaplastic in nature, with GM devoid of goblet cells and IM distinguished by goblet cells [13]. In GERD, chronic inflammation secondary to reflux of acidic gastric contents into the esophagus may lead to CM of the esophagus, with various mechanisms proposed [24,25]. Ultimately, BE, the only known precursor to esophageal adenocarcinoma, may develop [1].
According to the American Gastroenterological Association (AGA), the American College of Gastroenterology (ACG), and the European Society of Gastrointestinal Endoscopy (ESGE), the diagnosis of BE requires the presence of goblet cells, as IM is the only type of columnar epithelium of the esophagus found to pose a clearly significant cancer risk. This requirement for goblet cells has important implications for their guidelines on subsequent endoscopic surveillance, with no specific recommendations given for follow-up of GM of the esophagus [1,15–17]. However, the diagnostic criteria for BE have been widely debated. For example, guidelines from the Asian Pacific Association of Gastroenterology (APAGE), the British Society for Gastroenterology (BSG), and the Benign Barrett’s and Cancer Taskforce (BOB CAT) do not require goblet cells to diagnose BE [18–20].
Of note, there are also differences in opinion regarding optimal sampling, how to distinguish true goblet cells from pseudogoblet cells, and how to define the stage of disease; these ambiguities may interfere with detection of IM [8–14,26]. For example, detection of goblet cells has been shown to increase with the number of biopsies taken, with the length of columnar-lined esophagus, and with subsequent repeat endoscopy with biopsies [8–11,14]. Some also suggest that goblet cells actually develop later in disease progression [11–13,27]. In addition, a UK study actually found no significant difference in the subsequent development of dysplasia or adenocarcinoma between patients with IM versus those without IM at index endoscopy [11]. This was similar to another study wherein those with GM and those with specialized IM had a similar risk of esophageal cancer [28]. Even more confusing, some studies have shown that high goblet cell counts may be inversely related to the risk of adenocarcinoma in BE [29].
Three of the 5 cases in this case series did not demonstrate goblet cells, and one of these developed pseudogoblet cells after repeat endoscopy. Sampling error or potential future development of true goblet cells are possibilities in each of these cases. Detection of IM in these individuals may be missed without follow-up endoscopic evaluation. Furthermore, children such as these, with neurologic impairment or a history of esophageal atresia (EA), are at greater risk for severe GERD and development of Barrett’s esophagus during childhood than are otherwise healthy children [5].
In a recent retrospective study of 120 children with EA, there was 1 case of IM and 50 with GM [6]. In a second series of 209 children with EA, there were 4 with IM and 31 with GM. In this study, the cumulative prevalence of metaplasia increased to 15% after 15 years follow-up [7]. Recent guidelines from the GI working group of the International Network on Esophageal Atresia suggest that in asymptomatic patients with esophageal atresia, endoscopic surveillance begin after stopping PPI therapy, by 10 years of age, at transition to adulthood, and then systematically every 5–10 years, or with the development of any new esophageal symptoms or with worsening of existing esophageal symptoms [30]. However, in our series, one of the patients with a history of EA denied esophageal symptoms and developed Barrett’s esophagus by 8 years of age, despite his maintenance on PPI therapy since infancy. Of note, the above guidelines do not mention anemia as a symptom warranting endoscopic evaluation in those with EA, and they do not address surveillance endoscopy of gastric metaplasia in this population [30].
Also of note, a recent study following ACG guidelines for diagnosis of BE found that out of 7 children who were diagnosed with BE after repeat endoscopy, 4 had gastric metaplasia (ie, columnar metaplasia without goblet cells) at the time of prior endoscopic evaluation. Also, 6 of 9 children with intestinal metaplasia of the lower esophagus, but not meeting BE diagnostic criteria for length of extension, had prior findings of gastric metaplasia. Three of 6 of those with a short segment of intestinal metaplasia of the lower esophagus were later diagnosed with BE at follow-up endoscopic evaluation [31]. Similarly, another study found that 7 of 20 children with intestinal meta-plasia of the esophagus had only columnar metaplasia with no goblet cells at prior evaluation; the authors suggested that gastric metaplasia of the esophagus may be a precursor to BE in some children [32].
The cases presented in this series, along with the aforementioned issues and studies, highlight the need for specific guidelines for surveillance endoscopy of any form of columnar metaplasia in children with chronic GERD, such as those with neurodevelopmental delay. They also suggest the need for modification and further development of the current guidelines on evaluation and management of those with EA.
In this series, each patient had IDA as the sole or a primary presenting sign of gastric or intestinal metaplasia. The main finding of an inflamed, metaplastic lower esophagus in each case provided an explanation for chronic blood loss and consequent anemia, but malnutrition in the second case also likely contributed to that child’s anemia. One may question how to prove that the blood loss in these children originated from the region of metaplasia in the esophagus, especially when the fecal occult blood test (FOBT) was negative. While this area was markedly inflamed in all cases, highlighted by the blue-light evaluation and proven in the biopsies, esophageal blood loss may be missed with various fecal occult blood tests [33,34]. Nakama and colleagues showed the FOBT to be only 16.7% sensitive for detecting ulcerative esophagitis and 20% sensitive for esophageal cancer [35]. Therefore, the negative FOBTs for the first and third cases do not rule out esophageal CM with associated bleeding as the cause for their anemia. Tagged red blood cell scans (technetium-labeled erythrocyte scintigraphy) have notoriously poor negative and positive predictive values in acute GI bleeding and are not used to identify bleeding in chronic cases [36]. These patients had extensive evaluations, and in each case, the only site of significant inflammation was in the columnar-lined epithelium of the lower esophagus.
Our cases further demonstrate that the absence of GI symptoms should not preclude upper GI endoscopy in children with unexplained IDA, especially in those with neurologic impairment or EA. Of the 3 children with neurodevelopmental delay, 1 had recurrent pneumonias, but none had overt vomiting, hematemesis, or melena. Similarly, the 2 patients with EA presented with significant IDA but no reported GI symptoms. Endoscopy revealed striking findings and finally led to an explanation for otherwise unexplained IDA in each of these cases [37].
Conclusions
In summary, several key lessons arise from these cases. First, accurate detection of BE can be challenging, with important implications for future management of CM of the lower esophagus. Therefore, in children with severe neurologic impairment or a history of EA, who are particularly at an increased risk for BE due to severe GERD, guidelines are especially needed for the surveillance of columnar metaplasia of the esophagus, given the risk of malignant transformation, even in the absence of related IDA. Furthermore, IDA may be the primary presenting sign of GM or IM of the lower esophagus. Therefore, in children with neurologic impairment or a history of EA, upper GI endoscopy should be pursued in the evaluation of unexplained or refractory IDA, even in the absence of GI symptoms or positive fecal occult blood tests.
References:
1.. Spechler SJ, Sharma P, Souza RF, American Gastroenterological Association medical position statement on the management of Barrett’s esophagus: Gastroenterology, 2011; 140(3); 1084-91
2.. El-Serag HB, Gilger MA, Shub MD, The prevalence of suspected Barrett’s esophagus in children and adolescents: A multicenter endoscopic study: Gastrointest Endosc, 2011; 140(3); 1084-91
3.. Nguyen DM, El-Serag HB, Shub M, Barrett’s esophagus in children and adolescents without neurodevelopmental or tracheoesophageal abnormalities: A prospective study: Gastrointest Endosc, 2011; 73(5); 875-80
4.. Hsieh H, Frenette A, Michaud L, Intestinal metaplasia of the esophagus in children with esophageal atresia: J Pediatr Gastroenterol Nutr, 2017; 65(1); e1-4
5.. Hassall E, Co-morbidities in childhood Barrett’s esophagus: J Pediatr Gastroenterol Nutr, 1997; 25(3); 255-60
6.. Schneider A, Gottrand F, Bellaiche M, Prevalence of Barrett esophagus in adolescents and young adults with esophageal atresia: Ann Surg, 2016; 264(6); 1004-8
7.. Koivusalo AI, Pakarinen MP, Lindahl HG, Endoscopic surveillance after repair of oesophageal atresia: Longitudinal study in 209 patients: J Pediatr Gastroenterol Nutr, 2016; 62(4); 562-66
8.. Oberg S, Johansson J, Wenner J, Endoscopic surveillance of columnar-lined esophagus: Frequency of intestinal metaplasia detection and impact of antireflux surgery: Ann Sur, 2001; 234(5); 619-26
9.. Chandrasoma PT, Der R, Ma Y, Histologic classification of patients based on mapping biopsies of the gastroesophageal junction: Am J Surg Pathol, 2003; 27(7); 929-36
10.. Harrison R, Perry I, Haddadin W, Detection of intestinal metaplasia in Barrett’s esophagus: An observational comparator study suggests the need for a minimum of eight biopsies: Am J Gastroenterol, 2007; 102(6); 1154-61
11.. Gatenby PA, Ramus JR, Caygill CP, Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus: Scand J Gastroenterol, 2008; 43(5); 524-30
12.. Chandrasoma PT, Der R, Dalton P, Distribution and significance of epithelial types in columnar-lined esophagus: Am J Surg Pathol, 2001; 25(9); 1188-93
13.. Naini BV, Souza RF, Odze RD, Barrett’s esophagus: A comprehensive and contemporary review for pathologists: Am J Surg Pathol, 2016; 40(5); e45-66
14.. Jones TF, Sharma P, Daaboul B, Yield of intestinal metaplasia in patients with suspected short-segment Barrett’s esophagus (SSBE) on repeat endoscopy: Dig Dis Sci, 2002; 47(9); 2108-11
15.. Shaheen NJ, Falk GW, Iyer PG, ACG clinical guideline: Diagnosis and management of Barrett’s esophgus: Am J Gastroenterol, 2016; 111(1); 30-50
16.. Weusten B, Bisschops R, Coron E, Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement: Endoscopy, 2017; 49(2); 191-98
17.. Qumseya B, Sultan S, Bain P, ASGE guideline on screening and surveillance of Barrett’s esophagus: Gastrointest Endosc, 2019; 90(3); 335-59.e2
18.. Fitzgerald RC, di Pietro M, Ragunath K, British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus: Gut, 2014; 63(1); 7-42
19.. Fock KM, Talley N, Goh KL, Asia-Pacific consensus on the management of gastro-oesophageal disease: An update focusing on refractory re-flux diease and Barrett’s oesophagus: Gut, 2016; 65(9); 1402-15
20.. Bennett C, Moayyedi P, Corley DA, BOB CAT: A large-scale review and delphi consensus for management of Barrett’s esophagus with no dysplasia, indefinite for, or low-grade dysplasia: Am J Gastroenterol, 2015; 110(5); 662-82
21.. Cooper BT, Barbezat GO, Barrett’s esophagus: A clinical study of 52 patients: Q J Med, 1987; 62(238); 97-108
22.. Borrie J, Goldwater L, Columnar cell-lined esophagus: Assessment of etiology and treatment. A 22 year experience: J Thorac Cardiovasc Surg, 1976; 71(6); 825-34
23.. Janus J, Moerschel SK, Evaluation of anemia in children: Am Fam Physician, 2010; 81(12); 1462-71
24.. Hassall E, Barrett’s esophagus: New definitions and approaches in children: J Pediatr Gastroenterol Nutr, 1993; 16(4); 345-64
25.. Souza RF, From reflux esophagitis to esophageal adenocarcinoma: Dig Dis, 2016; 34(5); 483-90
26.. Wang H BI, Kumarasinghe P, Langner C, Poor agreement for detection of goblet cells in esophageal and GEJ biopsies [Abstract]: Mod Pathol, 2012; 25(Suppl. 2s); 184A-85A
27.. Lowes H, Somarathna T, Shepherd NA, Definition, derivation, and diagnosis of Barrett’s esophagus: Pathological perspectives: Adv Exp Med Biol, 2016; 908; 111-36
28.. Kelty CJ, Gough MD, Van Wyk Q, Barrett’s oesophagus: Intestinal metaplasia is not essential for cancer risk: Scand J Gastroenterol, 2007; 42(11); 1271-74
29.. Srivastava A, Golden KL, Sanchez CA, High goblet cell count is inversely associated with ploidy abnormalities and risk of adenocarcinoma in Barrett’s esophagus: PLoS One, 2015; 10(7); e0133403
30.. Krishnan U, Mousa H, Dall’Oglio L, ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula: J Pediatr Gastroenterol Nutr, 2016; 63(5); 550-70
31.. Putra J, Arva NC, Tan SY, Barrett esophagus and intestinal metaplasia of the gastroesophageal junction in children: A clinicopathologic study: J Pediatr Gastroenterol Nutr, 2020; 70(5); 562-67
32.. Sandys S, Loomes C, Keane A, Columnar lined esophagus/gastric metaplasia requires careful follow-up: J Pediatr Gastroenterol Nutr, 2020; 71(4); e136
33.. Harewood GC, McConnell JP, Harrington JJ, Detection of occult upper gastrointestinal tract bleeding: Performance differences in fecal occult blood tests: Mayo Clin Proc, 2002; 77(1); 23-28
34.. Rockey DC, Auslander A, Greenberg PD, Detection of upper gastrointestinal blood with fecal occult blood tests: Am J Gastroenterol, 1999; 94(2); 344-50
35.. Nakama H, Kamijo N, Fattah AS, Zhang B, Immunologic detection of fecal occult blood from upper digestive tract diseases: Hepatogastroenterology, 1998; 45(21); 752-54
36.. Tabibian JH, Wong Kee Song LM, Enders FB, Technetium-labeled erythrocyte scintigraphy in acute gastrointestinal bleeding: Int J Colorectal Dis, 2013; 28(8); 1099-105
37.. Naveed M, Dunbar KB, Endoscopic imaging of Barrett’s esophagus: World J Gastrointest Endosc, 2016; 8(5); 259-66
In Press
06 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942937
12 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943244
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943275
13 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943411
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250