29 November 2022: Articles 
Therapeutic Plasma Exchange and Supratherapeutic Levels of Unfractionated Heparin in the Management of Critically Ill Patient with Myasthenia Gravis: A Case Report
Unusual clinical course, Unusual or unexpected effect of treatment, Unexpected drug reaction
Shaden D. AlshehriDOI: 10.12659/AJCR.937617
Am J Case Rep 2022; 23:e937617
Abstract
BACKGROUND: Therapeutic plasma exchange (TPE) is an extracorporeal method of filtration indicated in several conditions, including myasthenia gravis (MG). The removal and replacement of plasma through TPE affect the level of coagulation factors, suggesting alterations in homeostasis. TPE also has the potential to remove medications from the plasma. Insufficient data are available that evaluate the effect of TPE on certain medications, such as unfractionated heparin (UFH).
CASE REPORT: We report a case of a 78-year-old woman with MG. She underwent a thymectomy complicated by phrenic nerve injury and respiratory failure, requiring admission to the Intensive Care Unit (ICU) and mechanical ventilation. She developed a provoked left upper extremity deep venous thrombosis and started on therapeutic UFH with a target activated partial thromboplastin time (aPTT) of 50 to 80 seconds. Despite being on immunosuppressants, additional therapy with TPE was deemed necessary for her MG exacerbation. Therefore, she received 5 sessions of TPE, given every other day. Interestingly, while on TPE therapy, the aPTT increased significantly after each administration, with TPE reaching >170 seconds in some instances. As a precautionary measure, heparin infusion was held for 1 day based on the institutional heparin protocol and the physician’s decision. Fortunately, the patient did not develop any bleeding complications.
CONCLUSIONS: TPE treatment may temporarily deplete the coagulation factors, leading to supratherapeutic aPTT levels. UFH dose adjustment and frequent assessment of aPTT levels are essential during TPE treatment to minimize serious bleeding complications. Future studies with a larger sample size are required to focus on understanding the effect of TPE on medications.
Keywords: Drug-Related Side Effects and Adverse Reactions, Heparin, Plasma Exchange, Female, Humans, Aged, Critical Illness, Plasmapheresis, Myasthenia Gravis
Background
Therapeutic plasma exchange (TPE) is a method implemented to filter high-molecular-weight or pathogenic substances (eg, antibodies, immune complexes, and endotoxins) from the plasma. TPE has other indications in neurological and non-neurological diseases, including myasthenia gravis (MG) and Guillain-Barre syndrome [1]. TPE is relatively safe and well-tolerated. However, the changes in plasma components due to TPE are unpredictable. Complications of TPE include hypocalcemia, QT prolongation, anaphylactoid reactions, and coagulopathy [2–4].
In addition to the removal of pathogenic substances, TPE also removes natural components of the plasma, suggesting alterations in homeostasis. It was reported in an earlier study that immediately after TPE, several coagulation parameters were prolonged, including prothrombin time (PT), partial thromboplastin time (aPTT), and thrombin time (TT). Other levels were decreased, including fibrinogen, platelet count, and antithrombin III. In addition, factors V, VII–X, IX, X, factor VIII antigen, and procoagulant cofactor were significantly decreased. They returned to normal levels within 4 hours, while factor IX, factor VIII procoagulant, and von Willebrand factor activities returned to normal levels within 24 hours [5]. Likewise, the effect of TPE on coagulation, particularly in patients receiving parenteral or oral anticoagulants, was evaluated in a systematic review that reported the prolongation of aPTT in patients actively on unfractionated heparin (UFH) and low-molecular-weight heparin [6]. In another study, TPE significantly led to abnormalities in several coagulation parameters, including prolonging the aPTT level from 28±3 to 45±8 seconds [7].
TPE can also potentially remove medications from the plasma [8,9]. Subsequently, the alteration of drug pharmacokinetic parameters caused by TPE can lead to alterations in drug efficacy and safety [10]. Much remains unknown about the effect of TPE on bleeding and thrombosis risk with the concurrent use of anticoagulants. Here, we describe a patient who experienced a significant fluctuation of aPTT after each TPE session while on therapeutic UFH. This case report was written according to the CARE guidelines [11].
Case Report
We report a case of a 78-year-old woman with a past medical history of MG. She underwent a thymectomy, complicated by phrenic nerve injury, innominate vein, superior vena cava injury, and bleeding. As a result, she was transferred to the Intensive Care Unit (ICU) after surgery. She was on mechanical ventilation for respiratory failure, which was thought to be related to her injury and MG exacerbation. On day 6 of her admission, she developed a provoked deep venous thrombosis in her left upper extremity, confirmed by Doppler ultrasound. Therapeutic UFH 25000 U was initiated with a rate of 1100 U/h to achieve a target aPTT range of 50 to 80 seconds. She was on pyridostigmine bromide 60 mg 4 times daily, prednisolone 20 mg once daily, and azathioprine 100 mg once daily, without significant improvement.
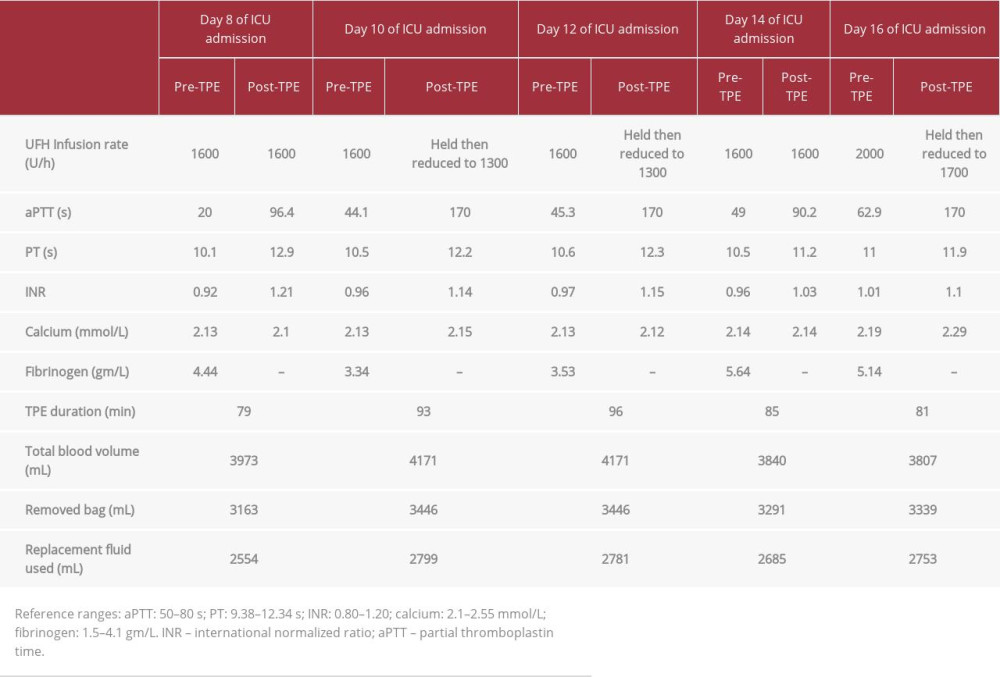
Although the patient was on immunosuppressants, additional therapy with TPE was deemed necessary. Therefore, she was scheduled to receive five sessions of TPE to be given every other day using a femoral Quinton catheter. The average duration of the TPE was 87 min (range 65–150 min), with 5% albumin used as replacement fluid and with approximately 3 L of exchanged volume for each procedure. Anticoagulation with UFH was used during the TPE sessions. On day 8, she received her first TPE session while on therapeutic UFH, with the same target level of aPTT. Interestingly, the aPTT increased (96.4 seconds) 4 hours after the TPE exchange. After 7 hours, the aPTT returned to the range (47.8 seconds). On day 10, she received her second TPE session, and the aPTT remarkably increased to >170 s; then, after 10 hours, it decreased to 48.4 s. The same pattern was seen after all TPE sessions, and she experienced fluctuations in her aPTT after each exchange (Figure 1). The UFH infusion rate was adjusted for each TPE session (Table 1).
During the hospital stay, the patient had no evidence of minor or major bleeding events, according to the International Society on Thrombosis and Haemostasis (ISTH) criteria. She showed clinical improvement and was discharged home on apixaban 5 mg orally twice daily. After a 3-month course of apixaban, she had a follow-up visit to assess her clinical status for her previous upper extremity deep venous thrombosis. She did not report any adverse bleeding or breakthrough clotting events while on apixaban. A Doppler ultrasound was negative, and apixaban was discontinued. Six months later, the patient had multiple follow-up visits for her MG. Fortunately, no thrombotic, minor, or major bleeding events were reported.
Discussion
Our patient experienced an abrupt supra-therapeutic aPTT immediately after the TPE session. It is well known that TPE depletes coagulation factors, as seen in our patient’s aPTT readings that increased temporarily and decreased with coagulation factor recovery, seen within 4 to 24 hours. The recovery of coagulation factors occurs in a biphasic pattern. It starts with a rapid recovery within the first 4 hours after exchange, followed by a slower increase between 4 and 24 hours after exchange [12]. It was reported that multiple treatments with short interval time between sessions produce more pronounced decrease in coagulation factors and can take several days to normalize [13]. Our findings appear to be in agreement with case reports and case series of patients who had TPE and had a rise in aPTT. Both studies support the hypothesis that TPE contributes to the loss of coagulation factors [14,15]. In addition, the concomitant anticoagulation effect of TPE and UFH on coagulation factors can cause major complications, such as bleeding, particularly in high-risk populations. In our case, despite the depletion of coagulation factors and the increase in aPTT, our patient did not experience major or minor bleeding as defined by ISTH criteria [16].
The intravascular volume removed by TPE must be replaced to prevent marked volume depletion. The replacement fluid used during therapeutic plasma exchange can be fresh frozen plasma, albumin, normal saline, or a combination of albumin and normal saline, based on clinical and laboratory findings. TPE with albumin as a replacement fluid is associated with a depletion of coagulation factors and prolongation of clotting times [12], which was observed in our case. Fresh frozen plasma, which has the advantage of containing clotting factors, is less likely to cause fluctuations in coagulation factor levels [1]. In addition, fluid replacement with albumin 5% is associated with hyperchloremic metabolic acidosis and depletion of coagulation factors as well as immunoglobulins [5,16]. Moreover, albumin replacement can decrease the fibrinogen level after TPE, causing hypofibrinogenemia, especially during the first 4 hours after TPE. The fibrinogen level can return to baseline 24 hours after TPE and, as a result, albumin replacement can prolong both aPTT/PT levels after TPE [5,16,17]. But in our case, only the aPTT level was prolonged, whilst the PT level was normal, which may indicate that the fibrinogen level was within the normal range after TPE. It is important to note that the fibrinogen level was not assessed after TPE in our patient.
aPTT is a screening blood coagulation test that measures the time in seconds of clot formation in the blood, which plays a major role in assessing the coagulation cascade pathway [18]. The clinical use of aPTT includes monitoring bleeding symptoms, diagnosing disseminated intravascular coagulation, and monitoring the effectiveness of UFH [18,19]. The target aPTT on a patient receiving UFH ranges from 50 to 80 seconds, based on our hospital laboratory value and protocol. A prolonged aPTT level with a normal PT level could occur for several reasons, such as the use of UFH, inherited deficiency of factor VIII, IX, or XI, acquired inhibitor of factor VIII, IX, XI, or XII, lupus anticoagulant, and acquired von Willebrand syndrome. On the other hand, prolongation of both aPTT and PT levels could occur as a result of hypofibrinogenemia, deficiency of prothrombin, factor V, or factor X [18–20]. The high aPTT could be explained by coagulation factor deficiency, which can lead to prolonged aPTT as a result of acquired inhibitors of factors VIII, IX, XI, or XII [18,19]. Some reports showed that depletion of factor XII due to its contact with the extracorporeal circuit could result in a prolongation of aPTT level after TPE [19,20].
Regional citrate and UFH are common anticoagulation agents used to prevent circulatory collapse and the loss of expensive blood components and to prevent bleeding [14,17]. Regional citrate can lead to hypocalcemia, a common adverse effect of TPE that occurs due to the binding of ionized free calcium by the citrate during the TPE [17]. Moreover, hypocalcemia can occur when fresh frozen plasma is used as transfusion replacement, due to the presence of diluted citrate that is used as anticoagulation to collect the blood [21]. In our case, UFH was used for anticoagulation during TPE, which resulted in no effect on calcium levels.
TPE’s effect on the clotting profile is suggested to be caused by AT-III depletion rather than by the drug being removed [22]. AT-III removal predisposes patients to thrombotic effects and decreases the anticoagulation effect of the UFH, which can lead to subtherapeutic aPTT level [23]. Data on bleeding and thrombotic events in patients receiving UFH and TPE have not been reported. Thrombosis and bleeding events were not studied in the only case series reporting the use of UFH and TPE by Kaplan et al [15]. The net effect of procoagulant and anticoagulant factors is unknown. The literature lacks data on the appropriate therapeutic management of anticoagulation during TPE. At our institution, aPTT is used for UFH monitoring, and neither UFH serum concentration nor AT-III is measured.
This is the first case report demonstrating the impact of TPE on aPTT levels in a critically ill patient with MG in an ICU setting receiving therapeutic UFH. In addition, this case highlights the importance of aPTT measurement with UFH before and after each TPE session, which can prevent the supratherapeutic effect of UFH and fatal consequences, such as bleeding. We acknowledge some limitations in this report. AT-III and UFH serum concentrations could have helped determine the cause of coagulopathy and discriminate between coagulation factor depletion, AT-III depletion, and UFH removal. Another limitation is the lack of some supporting laboratory tests for differential diagnostics; for example, thromboelastography, rotational thromboelastometry, post-TPE fibrinogen level, and post-TPE adjusted calcium level.
Conclusions
TPE treatment in a critically ill patient in an MG crisis may temporarily deplete the coagulation factors, leading to supratherapeutic aPTT levels. Therefore, UFH dose adjustment, frequent assessment of aPTT levels, and close monitoring of bleeding are essential during TPE treatment to minimize any serious bleeding complications. Future studies with a larger sample size are required to focus on understanding the effect of TPE on medications.
References:
1... Padmanabhan A, Connelly-Smith L, Aqui N, Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the Writing Committee of the American Society for Apheresis: The eighth special issue: J Clin Apher, 2019; 34; 171-354
2... Lu J, Zhang L, Xia C, Tao Y, Complications of therapeutic plasma exchange: A retrospective study of 1201 procedures in 435 children: Medicine (Baltimore), 2019; 98(50); e18308
3... Coirier V, Lesouhaitier M, Reizine F, Tolerance and complications of therapeutic plasma exchange by centrifugation: A single center experience: J Clin Apher, 2022; 37; 54-64
4... Dogra A, Rana K, Rathod C, Prakash S, Outcome of therapeutic plasma exchange in Myasthenia gravis patients: J Fam Med Prim Care, 2020; 9; 5971
5... Flaum MA, Cuneo RA, Appelbaum FR, The hemostatic imbalance of plasma-exchange transfusion: Blood, 1979; 54; 694-702
6... Hodulik KL, Root AG, Ledbetter LS, Onwuemene OA, Effects of therapeutic plasma exchange on anticoagulants in patients receiving therapeutic anticoagulation: A systematic review: Transfusion, 2019; 59; 1870-79
7... Thölking G, Mesters R, Dittrich R, Assessment of hemostasis after plasma exchange using rotational thrombelastometry (ROTEM): PLoS One, 2015; 10; e0130402
8... Mokrzycki MH, Kaplan AA, Therapeutic plasma exchange: Complications and management: Am J Kidney Dis, 1994; 23; 817-27
9... Cheng CW, Hendrickson JE, Tormey CA, Sidhu D, Therapeutic plasma exchange and its impact on drug levels: An ACLPS critical review: Am J Clin Pathol, 2017; 148; 190-98
10... Ibrahim RB, Balogun RA, Medications in patients treated with therapeutic plasma exchange: Prescription dosage, timing, and drug overdose: Semin Dial, 2012; 25; 176-89
11... Riley DS, Barber MS, Kienle GS, CARE guidelines for case reports: Explanation and elaboration document: J Clin Epidemiol, 2017; 89; 218-35
12... Chirnside A, Urbaniak SJ, Prowse CV, Keller AJ, Coagulation abnormalities following intensive plasma exchange on the cell separator: II. effects on factors I, II, V, VII, VIII, IX, X and antithrombin III: Br J Haematol, 1981; 48; 627-34
13... Kaplan AA, Halley SE, Plasma exchange with a rotating filter: Kidney Int, 1990; 38; 160-66
14... Usami K, Kinoshita T, Tokumoto K, Successful treatment of plasma exchange for severe cerebral venous thrombosis with thyrotoxicosis: J Stroke Cerebrovasc Dis, 2009; 18; 239-43
15... Kaplan A, Raut P, Totoe G, Management of systemic unfractionated heparin anticoagulation during therapeutic plasma exchange: J Clin Apher, 2016; 31; 507-15
16... Bauer PR, Ostermann M, Russell L, Plasma exchange in the Intensive Care Unit: A narrative review: Intensive Care Med, 2022; 48(10); 1382-96
17... Lemaire A, Parquet N, Galicier L, Plasma exchange in the Intensive Care Unit: Technical aspects and complications: J Clin Apher, 2017; 32; 405-12
18... Rasmussen KL, Philips M, Tripodi A, Goetze JP, Unexpected, isolated activated partial thromboplastin time prolongation: A practical mini-review: Eur J Haematol, 2020; 104; 519-25
19... Kamal AH, Tefferi A, Pruthi RK, How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults: Mayo Clin Proc, 2007; 82; 864-73
20... Bashawri L, Ahmed M, The approach to a patient with a bleeding disorder: For the primary care physician: J Fam Community Med, 2007; 14; 53-58
21... Ho KM, Leonard A, Risk factors and outcome associated with hypomagnesemia in massive transfusion: Transfusion, 2011; 51; 270-76
22... Ibrahim RB, Balogun RA, Medications and therapeutic apheresis procedures: Are we doing our best?: J Clin Apher, 2013; 28; 73-77
23... Sultan Y, Bussel A, Maisonneuve P, Potential danger of thrombosis after plasma exchange in the treatment of patients with immune disease: Transfusion, 1979; 19; 588-93
In Press
04 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.941835
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943042
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942578
05 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943801
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250