16 May 2023: Articles 
Clinically Aggressive Uterine Epithelioid Leiomyosarcoma with Rhabdomyoblastic Differentiation and High Proliferation Rate: A Case Report
Challenging differential diagnosis, Unusual setting of medical care, Rare disease
Ji Yeon Kim1DE, Bomi KimDOI: 10.12659/AJCR.939349
Am J Case Rep 2023; 24:e939349
Abstract
BACKGROUND: Leiomyosarcoma is the most common uterine sarcoma. Leiomyosarcoma is classified into conventional leiomyosarcoma, epithelioid leiomyosarcoma, and myxoid leiomyosarcoma. Leiomyosarcomas with rhabdoid features have been rarely reported. Herein, we report a case of uterine leiomyosarcoma with rhabdoid features.
CASE REPORT: A 58-year-old Korean woman presented with acute abdominal pain. Computed tomography and magnetic resonance imaging of the pelvis revealed a large solid mass in the posterior wall of the uterus that extended to the uterine cervix. The patient underwent total hysterectomy with bilateral salpingo-oophorectomy and tumorectomy. Microscopic and immunohistochemical examination of the tumor revealed leiomyosarcoma with rhabdoid features and high proliferation rate. Next-generation sequencing showed PI3K amplification and ERBB2 amplification. Postoperative abdominal and pelvic computed tomography performed 3 weeks after the operation showed a mass at the vaginal stump that was attached to the urinary bladder and rectum. The patient underwent pelvic exenteration of remnant vaginal stump, rectum, and urinary bladder with loop ileostomy, and was diagnosed with recurrent leiomyosarcoma. One month later, after the second operation, a 13-cm recurrent mass was noted on the computed tomography. Chemotherapy was not done and the patient died during supportive treatment 7 months after diagnosis.
CONCLUSIONS: This case, which is a uterine leiomyosarcoma with rhabdoid features and high proliferation rate, recurred very fast, within 1 month, and showed an aggressive clinical course. The molecular classification and postoperative therapy are not well established in uterine leiomyosarcomas. Further studies are required to clarify the clinical and pathological characteristics of leiomyosarcomas.
Keywords: Actins, Leiomyosarcoma, Myogenin, Recurrence, Rhabdomyosarcoma, Uterus, Female, Humans, Middle Aged, Neoplasm Recurrence, Local, Uterine Neoplasms, Cell Proliferation
Background
Leiomyosarcoma, one of the most common sarcomas arising in the uterus, is pathologically classified as conventional, epithelioid, or myxoid [1]. They sometimes contain unusual cellular components, such as rhabdoid cells or osteoclast-like giant cells [2]. However, their clinical characteristics are not well known because the number of reported cases is too low. Generally, the treatment of choice of uterine leiomyosarcoma is total hysterectomy with or without bilateral salpingo-oophorectomy, and chemotherapy or external beam radiation therapy is optional [3]. In case of advanced stage, systemic chemotherapy can be considered, although postoperative treatment has not been established [4]. Here, we present a case of uterine leiomyosarcoma with rhabdoid features that occurred in a 58-year-old woman and showed a highly aggressive clinical course.
Case Report
A 58-year-old Asian woman presented with lower abdominal pain that persisted for several days. The patient was a nulliparous woman, and the patient had started monthly hormonal therapy 6 months before menopause. The patient denied any history of gynecological disease or previous surgery. She had no urinary symptoms or vaginal discharge. Tenderness was detected in the lower abdomen. Ultrasonography and magnetic resonance imaging of the pelvis, which were performed in the referring hospital, revealed an 9.0×8.3 cm, exophytic, solid mass with hemorrhage and necrosis, centered at the uterine posterior wall, and extending to the uterine cervix (Figure 1A). The radiologic diagnosis was uterine cervical cancer or endometrial cancer. The tumor markers were as follows: squamous cell carcinoma antigen level was 0.6 ng/ml (normal range: 0–1.5 ng/ml). Other tumor markers had not yet been evaluated. Positron emission tomography revealed hypermetabolic lesions in the uterine wall, lymph nodes of the right anterior diaphragm, and right lobe of the thyroid. Papillary carcinoma of the thyroid was confirmed by fine-needle aspiration cytology. Total abdominal hysterectomy, bilateral salpingo-oophorectomy, left pelvic lymph node dissection, partial omentectomy, appendectomy, and pelvic adhesiolysis were also performed. The solid mass was located on the posterior wall of the uterine corpus and extended to the uterine cervix (Figure 1B). The mass did not invade the rectum or bilateral adnexa, although it was tightly adhered to it. The mass was ruptured, and multiple tumor masses were found in the cul-de-sac. The left pelvic lymph nodes were excised and enlarged to 2 cm in size. In the surgical field, the omentum, urinary bladder, and appendix were tumor-free.
The solid-mass lesion was centered on the uterine wall and did not infiltrate the endometrium (Figure 1C). In addition to the primary lesion, several solid masses were also found in the posterior cul-de-sac. Microscopically, the tumor was mainly composed of epithelioid pleomorphic cells admixed with short spindle cells. Cellularity had increased significantly. Spindle cells were arranged in a fascicular pattern, consistent with conventional leiomyosarcoma (Figure 2A). The tumor cells invaded the vascular lumen or formed lymphovascular emboli (Figure 2B). Rhabdoid cells were occasionally found, which showed eccentric nuclei with nucleoli and abundant cytoplasm (Figure 2C).
Strap cells were rarely observed, but cross-striations were absent (Figure 2D). The myxoid stroma was focally present. Extensive necrosis and hemorrhage were observed. Mitosis was highly active (30/HPF), and atypical mitosis was often observed (Figure 2E). There were no precancerous lesions, such as leiomyomas or endometrial stromal tumors. Myogenin was primarily expressed in the nuclei of the epithelioid and rhabdoid cells (Figure 2F). Desmin expression was focally present, mainly in the epithelioid and rhabdoid cells, and rarely in the spindle cells (Figure 2G). Smooth-muscle actin was focally expressed in the cytoplasm of the rhabdoid cells (Figure 2H), and CD10 was focally positive at the edge of the tumor tissue. Smooth-muscle myosin heavy chain, myoglobin, estrogen receptor, progesterone receptor, ckit, CD34, HMB-45, and S-100 tests were negative. Strong p53 expression was noted in the tumor cells, and c-erb B2 expression was found in less than 10% of tumor cells with complete membranous staining and mild intensity. The histological diagnosis was epithelioid leiomyosarcoma with rhabdoid features.
Next-generation sequencing revealed many tier 2 genetic variants, including MCL1 amplification, NTRK1 amplification, DDR2 amplification, PI3KCA amplification, EGFR3 amplification, CCND3 amplification, LATS1 mutation (c.1303C>T), BRAF amplification, KRAS amplification, TP53 mutation (c.524G>A), ERBB2 amplification, MEF2B amplification, CCNE1 amplification, and SRC amplification, without tier 1 genetic alteration. After discharge, the patient visited a clinic with vaginal discharge at postoperative 18 days and was admitted for antibiotic therapy with a clinical diagnosis of vaginal stump infection. Follow-up computed tomography of the gastrointestinal tract showed a mass mearing 8.8 × 7.7 cm, newly developed between the recto-sigmoid colon and urinary bladder within 1 month (Figure 3A). It had internal air that resulted from communication with the vaginal stump. Pelvic exenteration of the urinary bladder, vaginal stump, and rectum was performed. The recurrent tumor was tightly attached to the rectum or urinary bladder, but it did not invade them (Figure 3B). The histological and pathological findings were almost identical to those of the previous hysterectomy.
Two weeks after operation, a new 13.0 cm lesion was noted in the pelvic cavity on computed tomography of the pelvis (Figure 4A, 4B). The radiological diagnosis was pelvic abscess but recurrence could not be ruled out. The patient had not received any additional treatment such as chemotherapy or external beam radiation therapy and was transferred to another hospital. Two months later, computed tomography revealed new 10-m recurrent lesion in the pelvic cavity. The thyroid papillary carcinoma had been not treated because of the poor condition of the patient. During supportive treatment she died 7 months after diagnosis.
Discussion
This tumor was microscopically composed of epithelioid, rhabdoid, and spindle cells, and expressed both smooth-muscle and skeletal muscle markers. Pathological differential diagnoses included high-grade endometrial stromal sarcoma, pleomorphic rhabdomyosarcoma, extragastrointestinal stromal tumor, malignant melanoma, and extrarenal rhabdoid tumors [5]. Pure rhabdomyosarcoma rarely occurs in the uterus, especially in the uterine myometrium or cervix [6]. In this case, desmin and myogenin, specific markers of rhabdomyosarcoma, were focally expressed and not diffusely expressed. Extrarenal rhabdoid tumors are negative for smooth-muscle markers [7]. S-100, HMB-45, CD34, and c-kit negativity excluded perivascular epithelioid cell tumors, malignant melanomas, or extragastrointestinal stromal tumors.
Rhabdoid cells are skeletal muscle-like cells that are histologically round, polygonal, or strap-shaped, with eccentric nuclei and abundant eosinophilic cytoplasm containing fibrillary or hyaline cytoplasmic globules [8]. These cells are found in leiomyoma, malignant mixed Müllerian tumors, endometrial stromal sarcomas, extrarenal rhabdoid tumors, and leiomyosarcomas [8–10]. Rhabdoid cells in leiomyosarcomas or leiomyomas sometimes express both smooth and skeletal muscle markers [11,12]. It is assumed that rhabdoid cells appear in the early stage of rhabdomyoblastic differentiation compared to spindle cells because of the higher expression rate of myogenin and morphological similarities to rhabdomyoblasts [11,13]. Interestingly, the pathological findings of the rhabdoid cells in this case, including myogenin positivity, myoglobin negativity, and no cross-striation, were identical to those reported by Okubo et al [11]. They suggested that double expression of smooth-muscle actin and myogenin in leiomyosarcoma with partial rhabdomyoblastic differentiation can result from trans-differentiation or synchronous differentiation [11].
Chinag et al reported NR4A3-PGR fusion and PGR rearrangement in 4 and 2 epithelioid leiomyosarcomas, respectively, with rhabdoid features in 17 cases, and the genetic alterations were reported as absent in endometrial stromal tumors and perivascular epithelioid cell tumors [14]. PGR-rearranged tumors show diffuse or focal expression of desmin and diffuse positivity of estrogen and progesterone receptors, which differs from this case [14]. We did not evaluate genetic alterations, including PAX3-FOXO1 fusion, PAX7-POXO1 fusion, NR4A3-PGR fusion, PGR rearrangement YWHAE-NUTM2A/B fusions, BCOR rearrangement, or internal tandem duplications of BCOR, or SMARCA4 mutations, because the next-generation sequencing panels were designed for common solid tumors, not for uterine soft-tissue tumors [6,15–17]. This case showed some genetic alterations, including PI3KCA amplification, BRAF amplification, KRAS amplification, TP53 mutation (c.524G>A), and ERBB2 amplification, which may be critical for genetic classification or targeted therapy. According to Gao et al, TP53, RB, PI3K, and IDH1 mutations are the most common in sarcomas [18]. Uterine leiomyosarcoma is not well classified genetically, like endometrial stromal tumors. This case showed PI3KA amplification and ERBB2 amplification. Target therapy for uterine leiomyosarcoma is not standardized, and efforts to find treatment target are ongoing, focusing on molecular targets that have been proven in cancers of other organs. Ahmed et al evaluated the safety and efficacy of human epidermal growth factor receptor 2 (HER-2)-specific chimeric antigen receptor-modified T cells for immunotherapy of HER-2 positive sarcomas [19]. In uterine leiomyosarcomas, ERBB2 protein overexpression and amplification are rare, as 0.9% (1/106) ERBB2 expression was noted mainly in epithelial component of adenocarcinoma [20,21]. Cuppens et al suggested that dual PI3K/mTOR inhibition could be an effective treatment in uterine leiomyosarcomas and P-S6S240 can be a potential target for treatment [20].
Oshiro et al analyzed the clinical course of 133 cases of leiomyosarcoma with or without rhabdoid features and showed that the presence of rhabdoid cells is a poor prognostic factor in superficial soft-tissue leiomyosarcomas but not in retroperitoneal leiomyosarcomas [22]. We summarized the clinical (Table 1) and pathological (Table 2) characteristics of uterine leiomyosarcomas. Several cases, including the present case, showed an aggressive clinical course [11,12,23]. Therefore, the presence or absence of rhabdoid cells is not considered an independent marker of poor prognosis for uterine leiomyosarcomas. Mitotic count, extent of tumor necrosis, cytologic atypia, tumor size, and regional or distant metastasis are considered the main prognostic factors in uterine leiomyosarcomas [24]. Interestingly, clinically aggressive cases, including the present case, showed high mitotic rates. However, counting mitosis varies individually, and we think that assessment of the Ki-67 labeling index may be more accurate than mitotic count, especially measurement with image analysis, from the perspective of assessing the proliferation rate [25]. Travaglino et al reported that a Ki-67 labeling index of ³10% was a significantly poor prognostic factor in uterine leiomyosarcomas [26].
Conclusions
This case, which was a uterine leiomyosarcoma with rhabdoid features and high proliferation rate, recurred very fast and showed a highly aggressive clinical course. Molecular classification and postoperative treatment such as targeted therapy are not well established in uterine leiomyosarcomas. We summarized and compared uterine leiomyosarcoma cases that showed similar pathological findings to our case. Efforts to find a potential therapeutic target are ongoing and further studies are required to clarify the clinical and pathological characteristics of leiomyosarcoma.
Figures
References:
1.. Longacre TA, Lim D, Parra-Herran C, Uterine leiomiyosarcoma.: Female Genital Tumours: WHO Classification of Tumours (Medicine), 2020; 283-85, World Health Organization
2.. Terasaki M, Terasaki Y, Wakamatsu K, Uterine leiomyosarcomas with osteoclast-like giant cells associated with high expression of RUNX2 and RANKL: Virchows Arch, 2021; 478(5); 893-904
3.. Abu-Rustum NR, Yashar CM, Arend R: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Uterine Neoplasms., 2022, National Comprehensive Cancer Network [updated December 22, 2022; cited 2023 February 3]; 1.2023
4.. Hensley ML, Leitao MM, Treatment and prognosis of uterine leiomyosarcoma., 2023 [updated Oct 28, 2022; cited 2023 Feb 3]; Available from: https://www.uptodate.com/contents/treatment-and-prognosis-of-uterine-leiomyosarcoma
5.. Parra-Herran C, Howitt BE, Uterine mesenchymal tumors: Update on classification, staging, and molecular features.: Surg Pathol Clin, 2019; 12(2); 363-96
6.. Pinto A, Kahn RM, Rosenberg AE, Uterine rhabdomyosarcoma in adults: Hum Pathol, 2018; 74; 122-28
7.. Al-Hussaini M, Hirschowitz L, McCluggage WG, Uterine neoplasms composed of rhabdoid cells do not exhibit loss of INI1 immunoreactivity and are not related to childhood malignant rhabdoid tumor: Int J Gynecol Pathol, 2008; 27(2); 236-42
8.. Arora R, Abou-Bakr A, Albannai R, Uterine leiomyoma with peculiar skeletal muscle like and rhabdoid cells – case discussion and literature review: Kuwait Med J, 2015; 47(2); 149-52
9.. Sciallis AP, Bedroske PP, Schoolmeester JK, High-grade endometrial stromal sarcomas: A clinicopathologic study of a group of tumors with heterogenous morphologic and genetic features: Am J Surg Pathol, 2014; 38(9); 1161-72
10.. Gupta N, Dudding N, Smith JH, Eight cases of malignant mixed Mullerian tumor (carcinosarcoma) of the uterus: Findings in SurePath cervical cytology: Diagn Cytopathol, 2014; 42(2); 165-69
11.. Okubo Y, Shibuya K, Namiki A, Leiomyosarcoma with partial rhabdomyoblastic differentiation: First case report of primary cardiac origin: BMC Cancer, 2011; 11(1); 76
12.. Yorulmaz G, Erdogan G, Pestereli HE, Epithelioid leiomyosarcoma with rhabdoid features: Wien Klin Wochenschr, 2007; 119(17–18); 557-60
13.. Adhikari A, Kim W, Davie J, Myogenin is required for assembly of the transcription machinery on muscle genes during skeletal muscle differentiation: PLoS One, 2021; 16(1); e0245618
14.. Chiang S, Samore W, Zhang L, PGR gene fusions identify a molecular subset of uterine epithelioid leiomyosarcoma with rhabdoid features: Am J Surg Pathol, 2019; 43(6); 810-18
15.. Kolin DL, Dong F, Baltay M, SMARCA4-deficient undifferentiated uterine sarcoma (malignant rhabdoid tumor of the uterus) : A clinicopathologic entity distinct from undifferentiated carcinoma: Modern Pathology, 2018; 31(9); 1442-56
16.. Kubo T, Sugita S, Wada R, Uterine epithelioid leiomyosarcoma with c-kit expression and YWHAE gene rearrangement: A case report of a diagnostic pitfall of uterine sarcoma: Diagn Pathol, 2017; 12(1); 26
17.. Momeni-Boroujeni A, Chiang S, Uterine mesenchymal tumours: Recent advances: Histopathology, 2020; 76(1); 64-75
18.. Gao P, Seebacher NA, Hornicek F, Advances in sarcoma gene mutations and therapeutic targets: Cancer Treat Rev, 2018; 62; 98-109
19.. Ahmed N, Brawley VS, Hegde M, Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma: J Clin Oncol, 2015; 33(15); 1688-96
20.. Cuppens T, Annibali D, Coosemans A, Potential targets’ analysis reveals dual PI3K/mTOR pathway inhibition as a promising therapeutic strategy for uterine leiomyosarcomas – an ENITEC Group Initiative: Clin Cancer Res, 2017; 23(5); 1274-85
21.. Movva S, Wen W, Chen W, Multi-platform profiling of over 2000 sarcomas: Identification of biomarkers and novel therapeutic targets: Oncotarget, 2015; 6(14); 12234-47
22.. Oshiro Y, Shiratsuchi H, Oda Y, Rhabdoid features in leiomyosarcoma of soft tissue: With special reference to aggressive behavior: Mod Pathol, 2000; 13(11); 1211-18
23.. Hiraizumi Y, Kamoi S, Inde Y, A case of tumor lysis syndrome following chemotherapy for a uterine epithelioid leiomyosarcoma with focal rhabdomyosarcomatous differentiation: J Obstet Gynaecol Res, 2011; 37(7); 947-52
24.. Iasonos A, Keung EZ, Zivanovic O, External validation of a prognostic nomogram for overall survival in women with uterine leiomyosarcoma: Cancer, 2013; 119(10); 1816-22
25.. Stalhammar G, Robertson S, Wedlund L, Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer: Histopathology, 2018; 72(6); 974-89
26.. Travaglino A, Raffone A, Catena U, Ki67 as a prognostic marker in uterine leiomyosarcoma: A quantitative systematic review: Eur J Obstet Gynecol Reprod Biol, 2021; 266; 119-24
27.. Chauhan P, Mardi K, Rawat G, Leiomyosarcoma with rhabdoid differentiation arising from leiomyoma: A rare entity.: Arch Med Health Sci, 2017; 5(1); 252-54
Figures
Tables
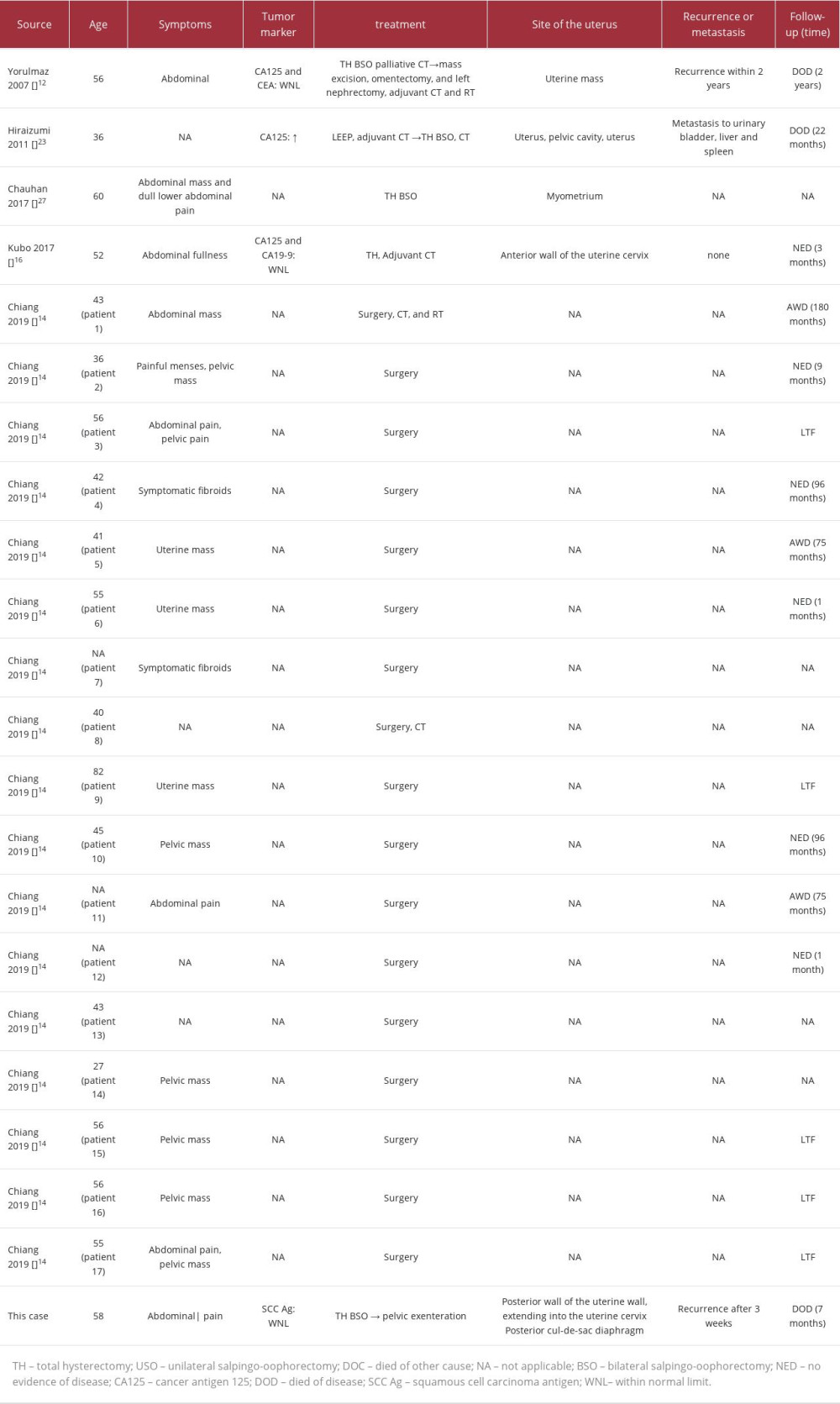 Table 1.. Summary of published cases of uterine leiomyosarcoma with rhabdoid features.
Table 1.. Summary of published cases of uterine leiomyosarcoma with rhabdoid features.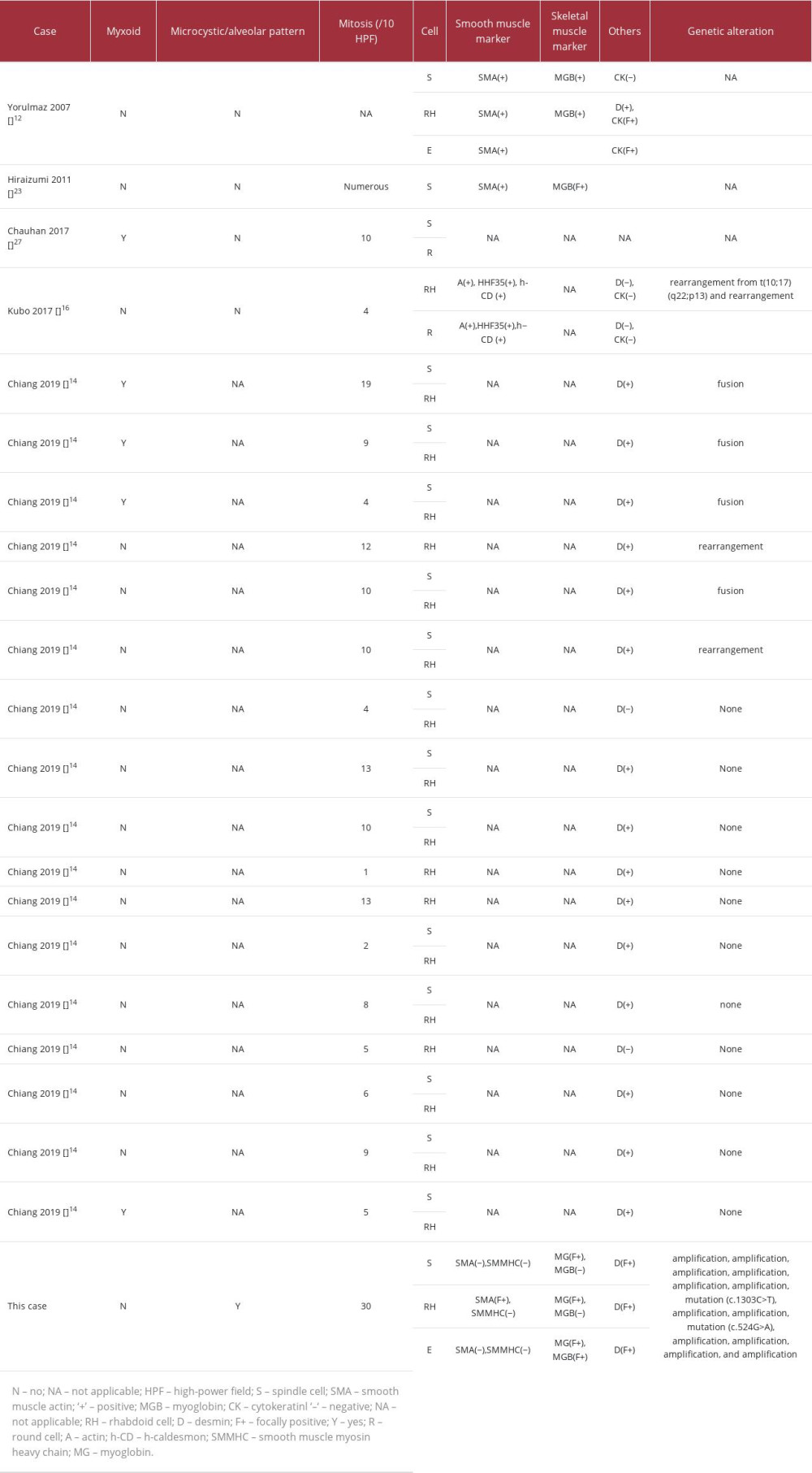 Table 2.. Summary of pathological and genetic findings of patients with leiomyosarcoma with rhabdoid differentiation.
Table 2.. Summary of pathological and genetic findings of patients with leiomyosarcoma with rhabdoid differentiation. Table 1.. Summary of published cases of uterine leiomyosarcoma with rhabdoid features.
Table 1.. Summary of published cases of uterine leiomyosarcoma with rhabdoid features. Table 2.. Summary of pathological and genetic findings of patients with leiomyosarcoma with rhabdoid differentiation.
Table 2.. Summary of pathological and genetic findings of patients with leiomyosarcoma with rhabdoid differentiation. In Press
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942826
14 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942770
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943214
16 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943010
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








