23 June 2023: Articles 
An Initially Missed Diagnosis of Venous Thromboembolic Phenomenon in Adult-Onset Still’s Disease: A Case Report and Literature Review
Unusual clinical course, Mistake in diagnosis, Diagnostic / therapeutic accidents, Management of emergency care, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis), Rare coexistence of disease or pathology
Mahlatse MankgeleDOI: 10.12659/AJCR.939520
Am J Case Rep 2023; 24:e939520
Abstract
BACKGROUND: Still’s disease is a rare multisystemic autoinflammatory disorder. The diagnosis of adult-onset Still’s disease (AoSD) can be challenging due to the rarity and overlapping features with many other systemic disorders. Complications of the illness can involve many systems in the human body. One of the least documented hematological complications of AoSD is thromboembolic phenomena.
CASE REPORT: This text outlines the presentation of a 43-year-old woman with a known diagnosis of AoSD, whose disease-modifying anti-rheumatic drugs (DMARDs) had been tapered and stopped due to remission. She presented with respiratory symptoms and features of an AoSD flare. Lack of complete improvement on antibiotic therapy and reinitiating of DMARDs prompted seeking an alternative/concurrent diagnosis. The work-up yielded a pulmonary embolism (PE) on the background of having no other risk factors for thrombosis.
CONCLUSIONS: In the reviewed literature, there is a close association between hyperferritinemia and AoSD complicated with venous thromboemboli (VTEs). A rigorous search for alternative diagnoses as well as other potential uncommon complications of AoSD is needed when working-up patients with AoSD, especially those that are not getting better on therapy. Given the rarity of AoSD, meticulous data collection may be useful in understanding the pathophysiology and features of presentation of the illness, including complications such as VTEs.
Keywords: Thrombosis, Still’s Disease, Adult-Onset, Adult, Female, Humans, Still's Disease, Adult-Onset, Missed Diagnosis, Antirheumatic Agents, Risk Factors, thromboembolism
Background
Adult-onset Still’s disease (AoSD) is analogous to systemic-onset juvenile idiopathic arthritis (SoJIA), which was initially described in the pediatric population by Sir George Frederic Still in the late 1800s [1]. AoSD primarily occurs in people aged 16–35 years, termed young adults, but there have been some reports in older individuals [1].
It is a rare systemic autoimmune disorder with an unclear cause, possibly having a genetic predilection as well as environmental triggers [2]. However, the pathophysiology stems from immune dysregulation causing an autoinflammatory cascade with resultant multiorgan-system involvement [3].
The hallmark features that are required to finalize the diagnosis are a quotidian fever, arthritis/arthralgia, rash and leukocytosis/neutrophilia [1]. There is no specific diagnostic test for AoSD, but there is a close association with elevated ferritin level >1000 ng/mL (specifically, non-glycosylated ferritin) [4]. AoSD has 3 main clinical courses: self-limiting, intermittent, and chronic [4].
The diagnosis of AoSD can be challenging due to the rarity and overlapping features with many other systemic disorders [3]. The 2 most used diagnostic criteria are the Yamaguchi criteria (96% sensitivity and 92% specificity) and the Fautrel criteria (87% sensitivity and 97.8% specificity) [4,5].
The complications of AoSD include the involvement of hematological, hepatic and cardiopulmonary organ-systems [1]. The most devastating complication of AoSD is reactive hemophagocytic lymphohistiocytosis (RHL), termed macrophage activation syndrome (MAS) in the setting of AoSD [1]. There are a handful of AoSD reported cases complicated with venous thromboembolic (VTE) phenomenona [6–9].
Here, we present a patient with intermittent AoSD who presented with a disease flare subsequently complicated with a pulmonary embolism (PE).
Case Report
Our patient was a 43-year-old woman residing in Johannesburg, South Africa. She was originally from Ethiopia. She has a gravidity and parity of 3, with uneventful pregnancies. She had in-situ an Etonogestrel implant (IMPLANON™) as contraception.
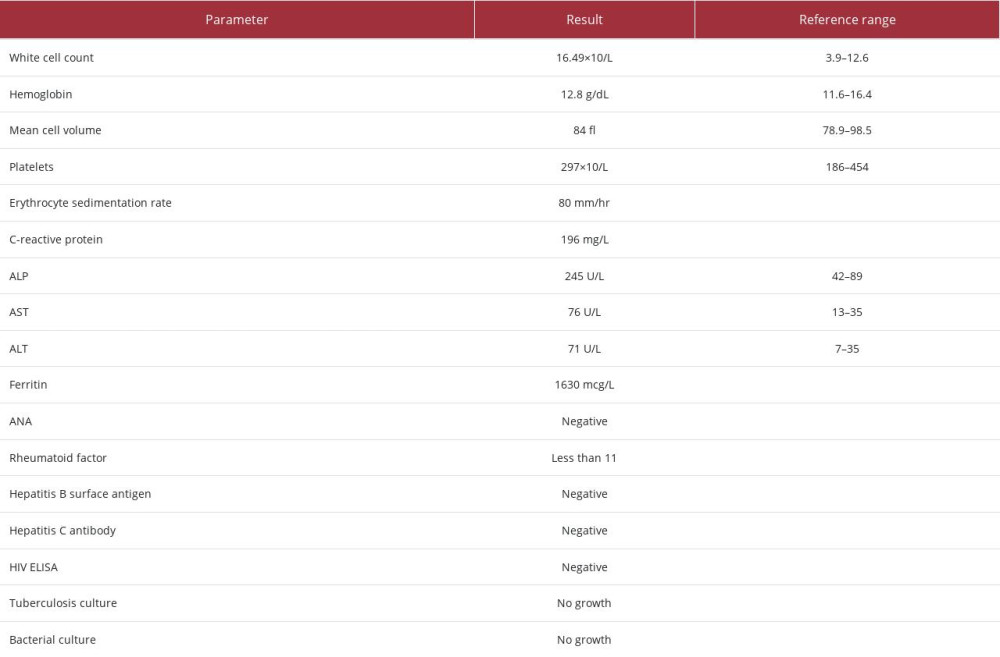
She was first referred to our department at the age of 33 in 2012 with a 10-year history of multiple recurrent episodes of pharyngitis, fever, chest pain, weight loss, loss of appetite, migratory arthralgia predominantly involving the hips; shoulders and knees, myalgia and a salmon-colored rash. Her symptoms were recurrent with periods of spontaneous resolution, consistent with intermittent AoSD. Her initial work-up is summarized in Table 1. A diagnosis of AoSD was made based on Yamaguchi criteria.
She was started on prednisone for the flare (subsequently tapered and ultimately stopped) and maintained on methotrexate. She developed methotrexate transaminitis in 2015 and was switched to cyclosporine. She had a planned pregnancy and delivered in 2019 without complications. Breastfeeding after the delivery necessitated halting cyclosporine therapy. After breastfeeding in 2019, disease-modifying anti-rheumatic drugs (DMARDs) were not reinitiated because of quiescent disease.
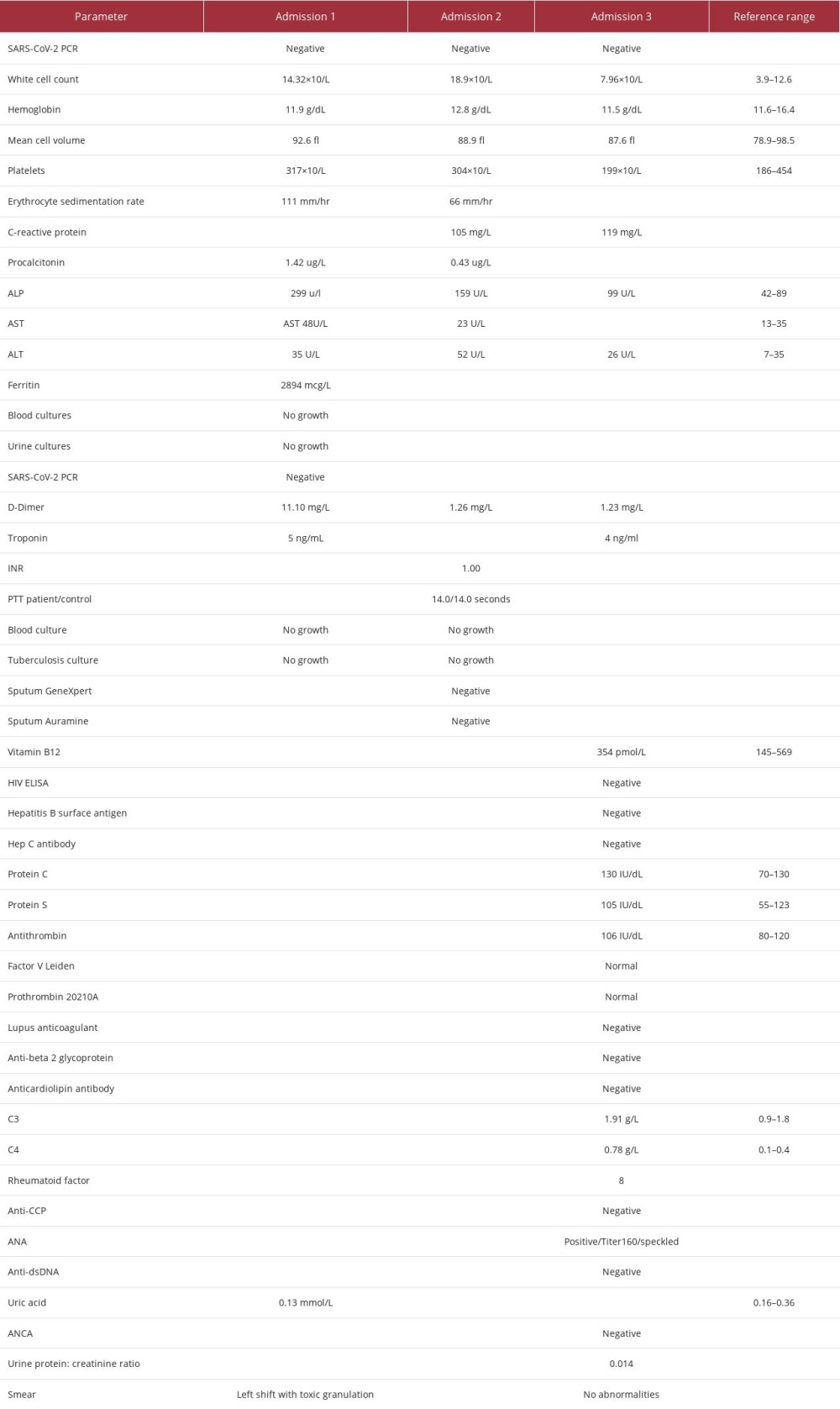
The patient was admitted 3 times between January and March 2022 with an AoSD flare that was complicated by a perplexing diagnosis of a PE. The patient was negative for COVID-19 on PCR in all 3 admissions.
In January she initially presented with fever, diaphoresis, rash on her anterior thighs and arms, polymyalgia, polyarthralgia, and a non-productive cough. On examination she was tachycardic, had a salmon-colored rash (arms and thighs), and had synovitis of the right knee and bilateral wrists. The work-up for this admission is summarized in Table 2, admission 1. Her chest X-ray is shown in Figure 1. A diagnosis of AoSD flare and community-acquired pneumonia was made. The AoSD flare was supported by a hyperferritinemia of 2894 ng/mL. She was treated with pulsed solumedrol therapy, completed a course of amoxicillin-clavulanic acid, and was started on cyclosporine as well as prednisone. On day 3 of her admission, she developed right-sided pleuritic chest pain that subsequently resolved 1 day later. A D-dimer done at this point was 11.1 mg/L. Her Well’s score at this point was 3 points, ie, 1.5 points for tachycardia and 1.5 points for reduced mobilisation from arthritis (moderate risk for PE). The chest pain and raised D-Dimer were attributed to be pleuritic chest pain related to the pneumonia. She was ultimately discharged.
Five days after discharge, she re-presented with massive hemoptysis, myalgia, tachycardia, and diffuse bi-basal coarse crackles. Her work-up for this second admission is summarized in Table 2, admission 2. Her Well’s score for this admission was 4.0 points, ie, additional 1 point for haemoptysis (moderate risk for PE). A computed tomography pulmonary angiogram (CTPA) showed no features of pulmonary thromboembolic disease. It also showed bilateral lung reticulonodular opacities with a tree-in-bud appearance. An infective process was deemed likely (Figure 2). Her gastroscopy was unremarkable. She was treated with piperacillin/tazobactam as a case of hospital-acquired pneumonia. She subsequently improved and was discharged.
The patient re-presented just over 1 month later with a 3-day history of left-sided pleuritic chest pain, not alleviated by analgesia. Her work-up for this third admission is summarized in Table 2, admission 3. Her electrocardiogram (ECG) had a P-pulmonale, Q3/T3, inverted T waves in V3-V6, and a sinus tachycardia (Figure 3). An echocardiogram showed a sinus tachycardia and normal right and left dimensions and function. A computed tomography (CT) scan of the chest was un-remarkable. A ventilation/perfusion (V/Q) scan had some areas of mismatch, which was diagnostic of a PE (Figures 4, 5). The diagnosis was also supported in retrospect by the initial D-dimer of 11.1 mg/L in admission 1. The patient was started on therapeutic Enoxaparin while a thrombophilia work-up was being done and she was ultimately initiated on Rivaroxaban. The thrombophilia work-up was unremarkable (Table 2, admission 3). In addition to blood and urinalysis, a malignancy screen, which included abdomen/pelvic ultrasound; mammogram and CT chest, was also unremarkable. She improved and was subsequently discharged on Rivaroxaban, Cyclosporine and Prednisone.
The patient was reviewed in our rheumatology clinic 2 weeks after discharge. She had improved respiratory symptoms.
Discussion
The most commonly described complications in the literature are hematological in the form of RHL/MAS, disseminated intravascular coagulopathy (DIC) and thrombotic microangiopathy (TMA), including thrombotic thrombocytopenic purpura (TTP) [1]. There are also reports of VTE in AoSD patients without any other identified risk factors for thrombosis.
Our team reviewed the literature published on VTEs and AoSD, as most of the literature on AoSD does not include it as a common complication of the disease. We performed a PubMed search using medical subject heading (MeSH) terms “(“Still’s Disease, Adult-Onset”[Mesh]) AND “Thrombosis”[Mesh]” without any filters and generated 3 relevant hits in the period 2005–2022 (20-year duration).
There are 3 cases of unprovoked PEs in AoSD, of which 2 were associated with an infective process and only 1 with no other notable cause for thrombosis that was isolated [6]. In all 3 cases, the patients had hyperferritinemia (a marker of inflammation), suggesting a potential for an inflammatory process being a risk factor for PE in patients with established AoSD [6].
In a review, Tamaki et al describe etiopathogeneses of VTE in systemic autoimmune diseases, especially in active disease [10]. The pathogeneses are either due to an increase in hypercoagulable factors or a lack of coagulation inhibitors [10]. The hyper-coagulable factors include but are not limited to increased tissue factor (TF) expression due to increased interleukin 6 (IL-6), platelet activation and neutrophil extracellular traps (NETs) created by a process called NETosis [10]. In terms of lack of inhibitors, there is reduced thrombomodulin due to increased tumour necrosis factor alpha (TNF-α) and increased interleukin 1 (IL-1) [10]. With the lack of inhibitors arm of the pathogeneses, there is also increased plasminogen activator inhibitor 1 (PAI-1) due to TNF-α, which inhibits the fibrinolytic system [10].
Other reported cases of VTEs that were not PEs include 1 patient with recurrent deep venous thrombosis (DVT), 1 with bilateral retinal arterio-venous thrombus occlusion, and 1 with porto-venous thrombosis on the background of AoSD [6–9].
An added confounder to the diagnosis of the PE in this particular patient was the variability of sensitivity and specificity of the tests used to make a diagnosis of a PE; the diagnosis of a PE was made on the third admission because of the infective/inflammatory processes that were overlapping and masking the diagnosis. The accuracy of diagnosis of a PE on CTPA decreases with more distal segmental emboli as the pulmonary arteries bifurcate, and the use of V/Q scan is more effective at detecting more peripheral segmental occlusions [11,12].
Conclusions
An acute flare of AoSD poses a clinical challenge when considering complications in certain organs, as it might have similar features to other inflammatory disorders, infective as well as malignant processes.
In the reviewed literature, there is a close association between hyperferritinemia and AoSD complicated with VTEs. A rigorous search for alternative diagnoses and other potentially uncommon complications of AoSD should be done when working-up patients with AoSD, especially those that are not improving on therapy. Given the rarity of AoSD, meticulous data collection may be useful in understanding the pathophysiology and features of presentation of the illness.
Figures
References:
1.. Mitrovic S, Feist E, Fautrel B, Adult-onset Still’s disease: Periodic and non-periodic fevers, 2020; 93-132, Switzerland, Springer Nature
2.. Leveille E, Nigrovic PA, Adult-onset Still’s disease. Updated: May 07, 2021 (13 screens) National Organization for Rare Disorders. Available from: https://rarediseases.org/rare-diseases/adult-onset-stills-disease/
3.. Tomaras S, Goetzke CC, Kallinich T, Feist E, Adult-onset Stil;’s disease: Clinical aspects and therapeutic approach: J Clin Med, 2021; 10(4); 733
4.. West SG: Rheumatology secrets, 2015, Philadelphia, PA, Elsevier/Mosby
5.. Lebrun D, Mestrallet S, Dehoux M, Validation of the Fautrel classification criteria for adult-onset Still’s disease: Semin Arthritis Rheum, 2018; 47(4); 578-85
6.. Bhamra M, Amarnani A, Ozeri D, Unprovoked pulmonary embolism identified on initial presentation of adult-onset Still disease in an elderly patient with no malignancy: J Clin Rheumatol, 2020; 26(2); e40-e42
7.. Jones RG, Hoyes B, Patel J, Zaman MO, Recurrent deep venous thromboses in a patient with adult-onset Still’s disease: BMJ Case Rep, 2021; 14(6); e240986
8.. Choudhry N, Vora RA, Diagnostic and therapeutic challenges: Retina, 2015; 35(7); 1480-83
9.. Morita H, Nishiwaki H, Nagayama Y, Yoshimura A, Portal vein thrombosis in adult-onset Still’s disease: A case report and literature review: Rheumatol Int, 2009; 29(12); 1515-18
10.. Tamaki H, Khasnis A, Venous thromboembolism in systemic autoimmune diseases: A narrative review with emphasis on primary systemic vasculitides: Vasc Med, 2015; 20(4); 369-76
11.. Bajc M, Schümichen C, Grüning T, EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond: Eur J Nucl Med Mol Imaging, 2019; 46(12); 2429-51
12.. Parker JA, Coleman RE, Grady E, SNM practice guideline for lung scintigraphy 4.0: J Nucl Med Technol, 2012; 40(1); 57-65
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250