02 September 2023: Articles 
A 65-Year-Old Man with Bilateral Adrenal Hemorrhage Following Prophylaxis for Postoperative Deep Vein Thrombosis with Rivaroxaban
Unusual clinical course, Challenging differential diagnosis
Safa Al-RawiDOI: 10.12659/AJCR.939816
Am J Case Rep 2023; 24:e939816
Abstract
BACKGROUND: Direct oral anticoagulant (DOAC) agents, such as rivaroxaban, treat and prevent venous thrombosis. Although adrenal hemorrhage due to DOACs has previously been reported, this is a rare condition that can present as an emergency. In this case report, we present a 65-year-old man who recently had bilateral knee arthroplasty and was started on rivaroxaban 10 mg daily for deep vein thrombosis (DVT) prophylaxis following the surgery.
CASE REPORT: Ten days after bilateral knee arthroplasty and starting rivaroxaban, the patient presented to the Emergency Department with severe, sudden abdominal pain. Abdominal computed tomography detected significantly enlarged bilateral adrenals, with ill-defined heterogeneous density extending to the upper part of perinephric and paranephric spaces, suggesting bilateral adrenal hemorrhage. A cosyntropin stimulation test was used to confirm the suspicion of adrenal insufficiency. Cortisol levels were 66 nmol/L before stimulation and 83 nmol/L 60 min after cosyntropin administration. Hydrocortisone was started intravenously at a dose of 50 mg every 8 h. After his symptoms improved, he was discharged on oral hydrocortisone at 10 mg in the morning and 5 mg in the evening. Seven weeks after discharge, follow-up abdominal ultrasonography showed that the bilateral adrenal hemorrhage had resolved.
CONCLUSIONS: This case supports previous cases of adrenal hemorrhage as a rare but serious association with rivaroxaban and highlights the importance of rapid diagnosis using imaging and monitoring of patients for this possible adverse effect. Practitioners must remain vigilant when prescribing anticoagulation therapy, especially in patients who are at an increased risk for adrenal hemorrhage.
Keywords: adrenal insufficiency, Arthroplasty, Replacement, Knee, Factor Xa Inhibitors, Male, Humans, Aged, Rivaroxaban, Cosyntropin, Hydrocortisone, Venous Thrombosis, Hemorrhage, Anticoagulants
Background
Adrenal hemorrhage (AH) is a rare adverse effect of anticoagulation, especially when involving both glands, with a predicted 15% mortality rate [1]. AH has vague symptoms, including abdominal pain, nausea, vomiting, neuropsychiatric symptoms, hypotension or shock, and fever. Without proper management, shock progresses to coma and death [2]. Its diagnosis is so challenging that it is often diagnosed at postmortem examination; however, the evolution of advanced imaging modalities enabled us to establish the diagnosis earlier in the clinical course [3]. Imaging, particularly computed tomography (CT), is the primary diagnostic tool for AH. The appearance of the hemorrhage on a CT scan is typically a round to ovoid lesion, sometimes accompanied by peri-adrenal fat stranding and bleeding extending into the peri-nephric space [4]. Fresh hematomas appear bright on CT scans, but their attenuation decreases over time. Ultrasonography is preferred for infants, owing to their small size and large adrenal glands. Contrast-enhanced ultrasound is used in pediatric patients to assess and monitor adrenal trauma, as well as to differentiate between AH and a mass. Magnetic resonance imaging (MRI) is considered the most accurate diagnostic modality, as it can distinguish between acute and chronic hematomas and detect underlying tumors [4].
Predisposing factors for AH include anticoagulant therapy, especially in cases of acute illness, underlying coagulopathy, septicemia, pregnancy, physical trauma, heparin-associated thrombocytopenia, and the postoperative state [2,5–7]. Unilateral AH is largely silent, whereas bilateral adrenal hemorrhage (BAH) is considered potentially fatal [8].
With the Food and Drug Administration (FDA)’s approval of the first direct oral anticoagulants (DOACs), dabigatran in 2010, rivaroxaban in 2011, apixaban in 2012, edoxaban in 2015, and betrixaban in 2017 [9], the range of anticoagulation alternatives has significantly increased over the past 10 years [9,10]. Compared with vitamin K anticoagulants, DOACs are less likely to interact with other drugs or foods, have a more stable pharmacokinetics profile, and do not need to be routinely monitored in the laboratory. Due to these advantages, DOACs have replaced vitamin K anticoagulants [11].
Dabigatran, rivaroxaban, apixaban, and edoxaban have FDA-approved indications for decreasing the risk of stroke and embolism in non-valvular atrial fibrillation, as well as in deep vein thrombosis and pulmonary embolism treatment and prophylaxis. Betrixaban is approved for preventing venous thromboembolism in hospitalized patients with an acute medical illness. Rivaroxaban combined with aspirin and clopidogrel is approved for the reduction of major cardiovascular events in patients with chronic coronary artery disease or peripheral artery disease, as well as postoperative thromboprophylaxis of deep vein thrombosis (DVT) in patients undergoing elective orthopedic surgery, such as knee and hip replacement surgery [12].
Several documented cases have shown a connection between the use of DOACs, specifically rivaroxaban, and AH. These cases, including reports by Alidoost et al in 2019 and Comuth et al in 2017 [8,13], highlight the rare but significant risk of AH associated with rivaroxaban. In a literature review by Sheklabadi et al in 2022 [14], two additional cases of AH following DOAC therapy including rivaroxaban were reported. These cases underscore the need for healthcare professionals to be aware of this potential complication and emphasize the importance of vigilant monitoring in patients receiving rivaroxaban or other DOACs. This report is of a 65-year-old man presenting with BAH following prophylaxis for postoperative DVT with rivaroxaban.
Case Report
We present the case of a 65-year-old man with a medical history of iron deficiency anemia who had recently undergone right knee arthroplasty. The patient presented to the Emergency Department (ED) with severe sudden left lower quadrant abdominal pain that lasted for 7 h and was accompanied by nausea and regurgitation. The pain was localized, continuous, severe, and burning in quality. It was unlike any pain he had experienced before. It was 9–10 out of 10 in severity on the numerical rating score. The patient denied fever, chills, vomiting, and urinary or bowel symptoms. Also, he denied a history of renal or gallbladder stones and a history of trauma.
Ten days prior, he underwent uncomplicated bilateral total knee arthroplasty due to bilateral severe knee osteoarthritis. The patient was started on the evening of the procedure on venous thromboembolism prophylaxis, dalteparin 7500 units for 5 days. He was discharged on a 35-day course of rivaroxaban 10 mg for DVT prevention. Owing to gastrointestinal bleeding concerns, the patient’s rivaroxaban was discontinued upon arrival in the ED, and he was kept on a below-the-knee pneumatic compression device.
Vital signs at the time of presentation were blood pressure of 160/89 mmHg, regular pulse of 88 beats/min, temperature 36.5°C, respiratory rate of 18 breath/min, and oxygen saturation of 99% on room air. Upon examination, the patient was in severe pain. Abdominal examination revealed tenderness, but with no rigidity or rebound tenderness. The Murphy sign was negative. The patient’s lower limbs were edematous and non-tender, with ecchymosis extending from the thigh to knees with knee dressing. A rectal examination done by an ED physician revealed no bleeding; the rectum was empty.
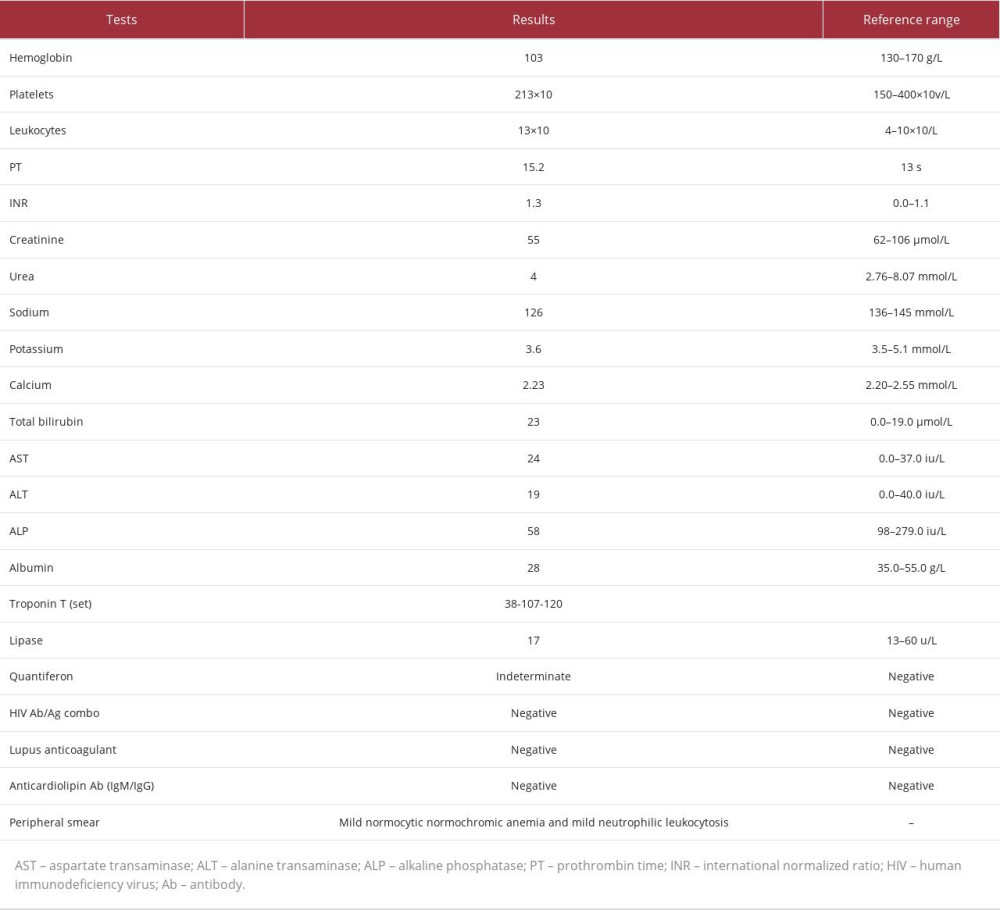
The initial laboratory investigation was notable for mild leukocytosis (white blood cells [WBC] 13×103/μL), normocytic normochromic anemia, and hyponatremia, while amylase and lipase levels were within the reference range. Table 1 presents the laboratory data of the patient. A random cortisol level at the time was 159 nmol/L.
A CT scan of the abdomen to rule out mesenteric ischemia, mass, collection, or acute pancreatitis identified significantly enlarged bilateral adrenals with ill-defined heterogeneous density extending to the upper part of the perinephric and paranephric spaces and nearby retroperitoneal structures, suggestive of BAH (Figure 1). The right adrenal gland measured 5×4.7×2.6 cm, and the left adrenal gland measured 5.2×4.2×2.8 cm; there was no CT evidence of thrombosis, occlusion, or drainable collection.
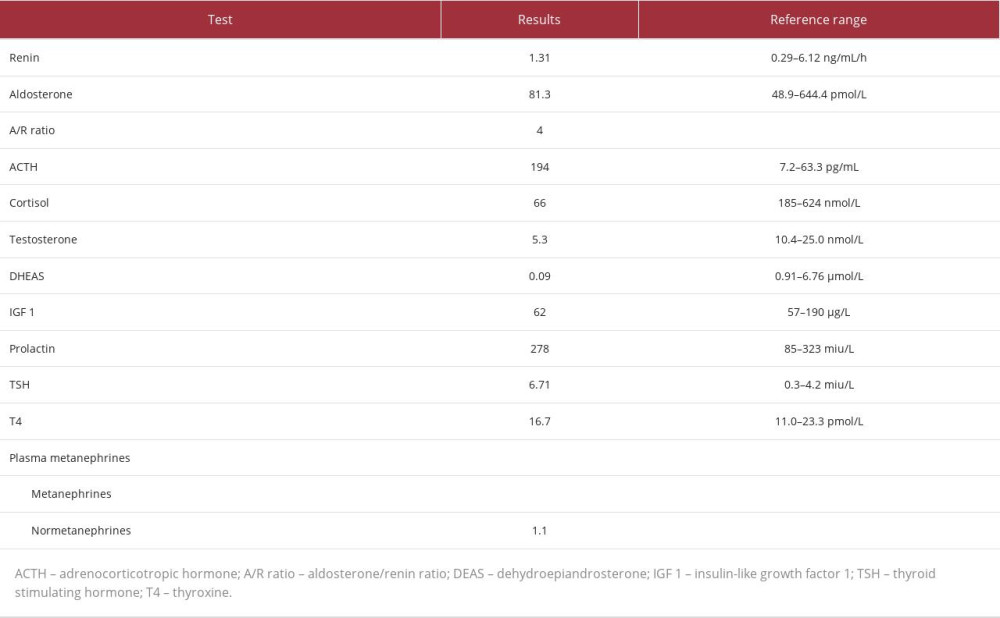
An endocrinologist was consulted and advised starting the patient empirically on hydrocortisone 50 mg intravenously 3 times a day, a repeat cortisol level test, and 60-min 250-mg adrenocorticotropic hormone stimulation (ACTH) test for adrenal insufficiency. The ACTH test was done 7 days after the first random cortisol level and was positive for adrenal insufficiency, with a low baseline cortisol-level of 66 nmol/L and a stimulated cortisol-level of 83 nmol/L (Table 2).
The electrocardiogram was unremarkable, with no ST-T wave changes or Q-wave. Echocardiography was done to rule out regional wall motion abnormalities, owing to a progressive increase in troponin T; however, it showed global hypercontractility of the left ventricle (LV) with an ejection fraction of 67% and grade 1 diastolic dysfunction. No regional wall motion abnormality was detected. Hyperdynamic kissing ventricular walls, with an intracavitary resting pressure gradient of 25 mm Hg, denoted under-filled LV that neither fit the criteria for diagnosing hyper-trophic obstructive cardiomyopathy nor stress cardiomyopathy.
After 2 days, the pain intensity improved, and the patient was weaned down to oral steroids, hydrocortisone 10 mg orally in the morning and 5 mg orally in the evening, and was discharged home without any oral anticoagulation. He was scheduled for repeat ultrasonography to evaluate the BAH. He was discharged home with close follow-up with his endocrinologist, internal medicine physician, and orthopedist. An abdominal ultrasound approximately 7 weeks after home discharge, done to monitor the hemorrhage and to look for any other structural lesions, demonstrated resolution of the BAH (Figure 2).
Echocardiography 8 months later showed normal global systolic LV function (ejection fraction 65%), with no intracavitary gradient, even with provocation with the Valsalva maneuver. During the study, the patient’s clinical status was settled; there was no pain and no volume depletion. This confirms the resolution of previous echocardiographic findings associated BAH.
Discussion
This case highlights the importance of recognizing and managing the potential complications of DOACs, specifically AH. Early recognition, prompt diagnosis, and appropriate management are essential to prevent serious consequences. Healthcare professionals should be aware of this rare but significant risk associated with the use of rivaroxaban and other DOACs. AH is a rare medical emergency, with a reported prevalence of 0.14% to 1.1% in postmortem investigations, although 15% of people who die from shock may have extensive BAH [15–17]. AH presents with non-specific signs and symptoms, making diagnosis quite challenging, with fever (66%) and abdominal or back pain (86%) being 2 consistent features of AH. Other symptoms include nausea and vomiting (47%), psychological symptoms (42%), and hypotension (19%), all of which are also common postoperative symptoms and can be missed or ignored [2]. Particularly, underlying physiological stress has been linked to nontraumatic AH, including sepsis, pregnancy, post-abdominal surgery, antiphospholipid syndrome, and, occasionally, anticoagulation [2,5–7].
Uncertainty surrounds the exact pathologic mechanism underlying spontaneous nontraumatic AH. However, it is thought to be related to the vascular structure of the adrenal gland, with a plexus of many arterioles and venules with limited drainage into a single vein. In times of stress, the ACTH secretion rises, which increases adrenal arterial blood flow that can surpass the organ’s limited venous drainage capacity, which predisposes the gland to hemorrhage [18]. Also, the high catecholamine levels secreted in a stressful state and adrenal vein thrombosis induced by coagulopathies can lead to adrenal vein spasm, stasis, and hemorrhage.
Pathological features typically include the involvement of bilateral glands, with extensive necrosis of all 3 cortical layers and medullary adrenal cells. Clinically, adrenal insufficiency is recognized when 90% of the gland is lost [3]. In patients with primary adrenal insufficiency, the main clinical symptoms of adrenal crisis are volume depletion and hypotension, primarily due to mineralocorticoid deficiency. Secondary or tertiary adrenal insufficiency (isolated glucocorticoid deficiency) have a somewhat higher blood volume, dilutional hyponatremia, less urinary sodium loss, and no hyperkalemia. Therefore, the adrenal crisis is less common in patients with secondary or tertiary adrenal insufficiency. An adrenal crisis is usually triggered by acute stress, acute cortisol deficiency due to pituitary infarction, or surgical treatment of Cushing’s syndrome [10]. Although our patient acquired primary adrenal insufficiency as a result of AH, he was found to have glucocorticoid and androgen insufficiency without mineralocorticoid deficiency, which can indicate an early presentation. However, approximately 50% of patients do not exhibit characteristic test abnormalities. Additionally, random cortisol levels used in the laboratory to diagnose adrenal insufficiency are unreliable, since reference values for patients under stress (such as those recovering from surgery) have not been thoroughly researched or defined. The coagulation profile is frequently within normal limits in patients with bilateral hemorrhage postoperatively, using prophylactic anticoagulants, and there is typically no sign of spontaneous bleeding elsewhere.
Although MRI or ultrasonography may also be used, the most effective and widely used imaging modality for diagnosis is CT. When there is a significant degree of clinical suspicion, repeat imaging is appropriate, because CT findings can be negative early during an AH. Bilateral adrenal hypertrophy and increased signal attenuation are indicators of BAH. MRI can be used to detect adrenal hematomas and assess their age [19,20].
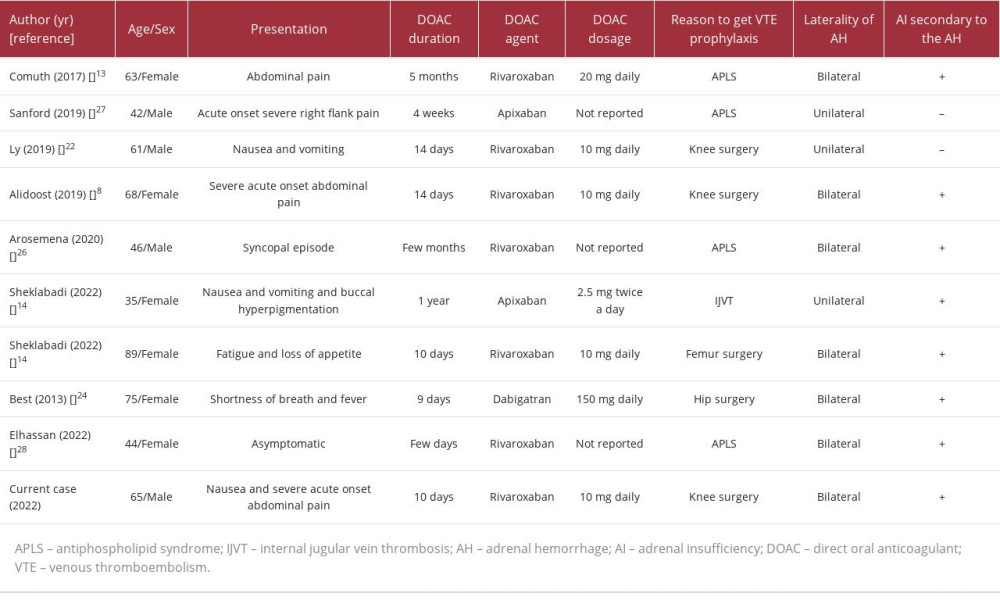
Without DVT prevention, it is thought that between 40% and 80% of patients undergoing joint replacement surgery will develop DVT [21]. Patients who were prescribed oral warfarin [21] and subcutaneous heparin for venous thromboembolism prevention following joint replacement surgery have been reported to develop BAH [21–24]. The first incidence of BAH caused by anticoagulant usage was recorded in 1947 [25]. Amador published the first report of successful resuscitation after the administration of corticosteroids in a patient with BAH brought on by the use of thromboprophylaxis in 1965 [25]. To the best of our knowledge, only 9 cases of AH have been linked to the use of DOAC medications (Table 3) [8,13,14,22,24,26–28]. Dabigatran was used in 1 case, apixaban in 2, and rivaroxaban in 6 cases. An article by Alidoost et al presents the most similar case to ours in the search for DOAC’s etiology of AH. It describes a 68-year-old woman who had undergone recent right knee arthroplasty and was taking 10 mg of rivaroxaban daily for 14 days as prophylaxis for DVT. She arrived at the ED with sudden and severe abdominal pain. An abdominal CT scan showed a potential left AH, which was later confirmed by MRI. A subsequent CT scan revealed a conversion from unilateral AH to BAH, leading to the development of adrenal insufficiency in the patient [8].
Adrenal insufficiency was present in every case of BAH we analyzed. In unilateral AH, the contralateral adrenal gland can become exhausted, resulting in hypocortisolemia, as seen in cases described by Sheklabadi et al and Ly and Quintero [14,22]. Three of the cases were associated with antiphospholipid syndrome, and 3 occurred due to prophylactic anticoagulation given to the patients after knee surgery. BAH is typically reported in patients between the ages of 61 and 82 years on days 4 to 10 following surgery, with a peak at days 6 to 7. It occurs more frequently following total knee replacement than following total hip replacement [21,23]. Kovacs et al conducted a multicenter case-control study to evaluate potential risk factors for the emergence of massive BAH [18]. In the multivariate analysis, thrombocytopenia, heparin exposure, and sepsis were reported to be strongly linked with the likelihood of bleeding. Heparin was used in 16 of the 23 patients with significant BAH, and 6 patients took it only subcutaneously. Only 2 patients were taking non-heparin anticoagulants or thrombolytics. The authors concluded that patients who were administered heparin via any route for 4 to 6 days and patients who were exposed for more than 6 days were, respectively, between 17 and 34 times more prone to experience BAH than individuals exposed for fewer than 4 days or not at all. Our patient was exposed to 10 days of rivaroxaban.
Survivors of postoperative AH and insufficiency have a good prognosis with corticosteroid replacement. Only a few studies have evaluated the long-term follow-up of glucocorticoid and mineralocorticoid function in individuals with BAH [29–34]. Four patients with acute BAH and adrenal insufficiency were followed for up to 19 years and revealed no requirement for long-term mineralocorticoid supplementation. They also showed that the serum cortisol levels of 3 of the 4 patients had improved, and 1 patient had been able to function without cortisol replacement, for 4 years. In our case, we found the need for continued adrenocortical replacement therapy after 18 months of follow-up. According to the Mayo Clinic study, with therapy, survival was 100% as opposed to 17% without it. However, despite effective medication, sepsis- or stress-related adrenal insufficiency leads to poor outcomes (survival with therapy was 9% against 6% without treatment) [3]. If the symptoms are left untreated, death can happen within hours to days.
The LV intracavitary pressure gradient detected by echocardiography at presentation is due to hypercontractility and under-filling of the LV, caused by bleeding and heightened sympathetic tone, secondary to acute stress and significant pain. This is supported by the presence of sinus tachycardia recorded during this echocardiographic study and the resolution of the intracavitary pressure gradient in the subsequent study done when the patient’s clinical status was settled. Neither echocardiography done at the time of admission nor follow-up echocardiography done 8 months later showed typical features of hypertrophic obstructive cardiomyopathy or stress cardiomyopathy. The unique morphology of stress-induced cardiomyopathy in the echocardiography study includes apical ballooning and the relative compensatory hypercontractility of the basal segments. The Venturi effect around the dynamic LV outflow tract, caused by basal hyper-contractility, results in the movement of the anterior mitral leaflets toward the interventricular septum in the systolic phase, “systolic anterior motion”, creating LV outflow tract obstruction [35]. These specific echocardiographic features were lacking in the 2 echocardiographic studies of our case. The progressive elevation of troponin T, in the absence of chest pain and ST-T wave changes, in addition to the absence of Q-wave in electrocardiogram and absence of regional wall motion abnormalities in echocardiography, did not fulfill the diagnostic criteria for ischemic myocardial injury; instead, it denoted acute nonischemic myocardial injury, according to the fourth universal definition of myocardial infarction [36]. This acute nonischemic myocardial injury was secondary to anemia, AH, and acute illness with accompanying stress.
It is intriguing that the risk of cardiovascular disease is increased not just by overtreatment [37–39] but also by transient adrenal insufficiency [40]. According to reports, the levels of inflammatory mediators, including interleukin-1, interleukin-6, and tumor necrosis factor, correlate with cortisol, and excessive levels of these mediators can be linked to a higher risk of cardiovascular events. Also, it has been suggested that glucocorticoid deficiency is linked to poor expression of K+ channels in the heart ventricles and dysfunction of the Ca2+ transporter in the heart membrane, diminishing the cardioprotective impact. Additionally, it has been suggested that glucocorticoid deficit results in hypothyroidism, which is known to be linked to cardiovascular issues.
Conclusions
BAH following surgery is uncommon and potentially fatal. This case supports previous cases of AH as a rare but serious association with rivaroxaban. It highlights the importance of rapid diagnosis using imaging and of monitoring of patients for this possible adverse effect. Reversing coagulopathies and initiating steroid replacement treatment early have the potential to save lives. Owing to the vagueness and lack of specificity of the clinical presentation and laboratory findings of AH, which resembles other non-life-threatening postoperative complications, making the diagnosis exceedingly tricky. An initial CT scan can be normal, further complicating the diagnosis. Therefore, healthcare professionals should be on high alert for associated complications in postoperative patients, even when administering prophylactic doses of anticoagulants, especially in older patients, who are at greater risk of bleeding.
Figures
References:
1.. Tormos LM, Schandl CA, The significance of adrenal hemorrhage: Undiagnosed Waterhouse-Friderichsen syndrome, a case series: J Forensic Sci, 2013; 58(4); 1071-74
2.. Rao RH, Vagnucci AH, Amico JA, Bilateral massive adrenal hemorrhage: Early recognition and treatment: Ann Intern Med, 1989; 110(3); 227-35
3.. Vella A, Nippoldt TB, Morris JC, Adrenal hemorrhage: A 25-year experience at the Mayo Clinic.: Mayo Clin Proc., 2001; 76(2); 161-68
4.. Mehmood KT, Sharman T, Adrenal hemorrhage.: StatPearls [Internet]., 2023, Treasure Island (FL), StatPearls Publishing https://www.ncbi.nlm.nih.gov/books/NBK555911/
5.. Xarli VP, Steele AA, Davis PJ, Adrenal hemorrhage in the adult.: Medicine (Baltimore), 1978; 57(3); 211-21
6.. Warkentin TE, Safyan EL, Linkins L-A, Heparin-induced thrombocytopenia presenting as bilateral adrenal hemorrhages: N Engl J Med, 2015; 372(5); 492-94
7.. Lehrberg A, Kharbutli B, Isolated unilateral adrenal gland hemorrhage following motor vehicle collision: A case report and review of the literature: J Med Case Rep, 2017; 11(1); 358
8.. Alidoost M, Soomro R, Gubeladze A, Rivaroxaban related bilateral adrenal hemorrhage: A rare complications of direct oral anticoagulants – a case reports: Am J Case Rep, 2019; 20; 917780
9.. Kustos S, Fasinu P, Direct-acting oral anticoagulants and their reversal agents – an update: Medicines, 2019; 6(4); 103
10.. Gunasekaran K, Rajasurya V, Devasahayam J, A review of the incidence diagnosis and treatment of spontaneous hemorrhage in patients treated with direct oral anticoagulants: J Clin Med, 2020; 9(9); 2984
11.. Barnes GD, Lucas E, Alexander GC, Goldberger ZD, National trends in ambulatory oral anticoagulant use: Am J Med, 2015; 128(12); 1300-5
12.. Singh R, Emmady PD, Rivaroxaban.: StatPearls [Internet]., 2023, StatPearls Publishing LLC https://www.ncbi.nlm.nih.gov/books/NBK557502/
13.. Comuth W, Christiansen JJ, Bloch-Munster AM, Husted S, Bilateral adrenal gland hemorrhage in a patient treated with rivaroxaban.: Blood Coagul Fibrinolysis, 2017; 28(1); 102-4
14.. Sheklabadi E, Sharifi Y, Tabarraee M, Adrenal hemorrhage following direct oral anticoagulant (DOAC) therapy: Two case reports and literature review: Thromb J, 2022; 20(1); 39
15.. Simon DR, Palese MA, Clinical update on the management of adrenal hemorrhage: Curr Urol Rep, 2009; 10(1); 78-83
16.. Gavrilova-Jordan LP, Edmister WB, Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management: Obstet Gynecol Surv, 2005; 60(3); 191-95
17.. Seow YTN, Ng ZQ, Wong SL, Anticoagulation-induced unilateral adrenal haemorrhage and pseudoaneurysm: BMJ Case Rep, 2019; 12(12); e232539
18.. Kovacs KA, Lam YM, Pater JL, Bilateral massive adrenal hemorrhage: Assessment of putative risk factors by the case-control method: Medicine (Baltimore), 2001; 80(1); 45-53
19.. Kawashima A, Sandler CM, Ernst RD, Imaging of nontraumatic hemorrhage of the adrenal gland: Radiographics, 1999; 19(4); 949-63
20.. Hoeffel C, Legmann P, Luton JP, Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases: J Urol, 1995; 154(5); 1647-51
21.. Park KJ, Bushmiaer M, Barnes CL, Bilateral adrenal hemorrhage in a total knee patient associated with enoxaparin usage: Arthroplast Today, 2015; 1(3); 65-68
22.. Ly BA, Quintero L, Adrenal insufficiency from unilateral adrenal hemorrhage in a patient on rivaroxaban thromboprophylaxis: AACE Clin Case Rep, 2019; 5(1); e70-e72
23.. Barrou Z, Verny C, Cohen-Bittan J, Verny M, Bilateral adrenal necrosis after knee arthroplasty: J Am Geriatr Soc, 2010; 58(11); 2248-49
24.. Best M, Palmer K, Jones QC, Wathen CG, Acute adrenal failure following anticoagulation with dabigatran after hip replacement and thrombolysis for massive pulmonary embolism.: BMJ Case Rep., 2013; 2013 bcr-2012-007334
25.. Amador E, Adrenal hemorrhage during anticoagulant therapy. A clinical and pathological study of ten cases.: Ann Intern Med, 1965; 63(4); 559-71
26.. Arosemena MA, Rodriguez A, Ediriweera H, Bilateral adrenal haemorrhage secondary to rivaroxaban in a patient with antiphospholipid syndrome: BMJ Case Rep, 2020; 13(7); e234947
27.. Sanford Z, Nanjundappa A, Annie FH, Embrey S, Adrenal hemorrhage in a patient anticoagulated with apixaban with antiphospholipid syndrome: Cureus, 2019; 11(7); e5108
28.. Elhassan YS, Ronchi CL, Wijewickrama P, Baldeweg SE, Approach to the patient with adrenal hemorrhage: J Clin Endocrinol Metab, 2022; 108(4); 995-1006
29.. Caron P, Definitive adrenal insufficiency due to bilateral adrenal hemorrhage and primary antiphospholipid syndrome: J Clin Endocrinol Metab, 1998; 83(5); 1437-39
30.. Delhumeau A, Moreau X, Chapotte C, Heparin-associated thrombocytopenia syndrome: An underestimated etiology of adrenal hemorrhage: Intensive Care Med, 1993; 19(8); 475-77
31.. Vengrove MA, Amoroso A, Reversible adrenal insufficiency after adrenal hemorrhage: Ann Intern Med, 1993; 119(5); 439
32.. Feuerstein B, Streeten DHP, Recovery of adrenal function after failure resulting from traumatic bilateral adrenal hemorrhages: Ann Intern Med, 1991; 115(10); 785-86
33.. Taylor HC, Sachs CR, Primary aldosteronism: Remission and development of adrenal insufficiency after adrenal venography: Ann Intern Med, 1976; 85(2); 207-9
34.. Jahangir-Hekmat M, Taylor HC, Levin H, Adrenal insufficiency attributable to adrenal hemorrhage: Long-term follow-up with reference to glucocorticoid and mineralocorticoid function and replacement: Endocr Pract, 2004; 10(1); 55-61
35.. Citro R, Lyon AR, Meimoun P, Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: Clinical and prognostic implications: J Am Soc Echocardiogr, 2015; 28(1); 57-74
36.. Zamorano JL, Anastasakis A, Borger MA, 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC): Eur Heart J, 2014; 35(39); 2733-79
37.. Ahmed M, Maliyakkal AM, Non-ST-segment elevation myocardial infarction shortly after starting steroid replacement therapy in a patient with adrenal insufficiency: Cureus, 2022; 14(5); 1-7
38.. Chang SS, Liaw SJ, Bullard MJ, Adrenal insufficiency in critically ill emergency department patients: A Taiwan preliminary study: Acad Emerg Med, 2001; 8(7); 761-64
39.. Shokr M, Rashed A, Lata K, Kondur A, Dexamethasone associated ST elevation myocardial infarction four days after an unremarkable coronary angiogram – another reason for cautious use of steroids: A case report and review of the literature: Case Rep Cardiol, 2016; 2016; 4970858
40.. Otsuka Y, Harada K, Yasuda M, Coronary spastic angina induced by adrenal insufficiency: Intern Med, 2020; 59(15); 1873-77
Figures
In Press
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942921
22 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943346
24 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943560
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250