27 July 2023: Articles 
The Role of Aquaporin 1 in Water Retention Mechanism of Arachnoid Cysts: Insights from 3 Case Reports
Unknown etiology, Challenging differential diagnosis
Yasutaka Kurokawa12ABCDEF*, Masanori Ishiguro3BC, Takayuki KurokawaDOI: 10.12659/AJCR.939834
Am J Case Rep 2023; 24:e939834
Abstract
BACKGROUND: Although arachnoid cysts are common lesions, the pathogenesis of their continuous growth remains unclear. We aimed to identify the role of aquaporins in arachnoid cyst specimens.
CASE REPORT: We selected 3 cases from our own facility and examined arachnoid cyst wall specimens, which were sampled intraoperatively. Patients presented with variable symptoms, a 52-year-old man with a “heavy sensation” in the head and dysesthesia on the left hand, a 68-year-old man with unsteady gait, and finally a 26-year-old woman with a history of intermittent headaches for 10 years. Intraoperative specimens were obtained and examined. Evaluation techniques were light microscopy, immunohistochemical staining for aquaporin, and electron microscopy. Light microscopy showed that cells were arranged in epithelium-like structures forming several thick lamellae, with visible connective tissue among them. Under electron microscopic examination, cells with many or few cell organelles and with spindle-like nuclei were arranged in lamellar or flattened structures. Many vacuolizations were seen in between. Interdigitation of cells and many desmosomes were observed. All 3 cases were positive for aquaporin 1.
CONCLUSIONS: Our study showed that water transportation through aquaporin 1 has a potential role in the formation and expansion of arachnoid cysts.
Keywords: Aquaporin, Arachnoid Cysts, Cerebrospinal Fluid, Immunohistochemistry, Male, Female, Humans, Adult, Middle Aged, Aged, Aquaporin 1, Water-Electrolyte Imbalance, Microscopy, Electron, Gait Disorders, Neurologic
Background
Arachnoid cysts, summarized and reported as serous cysts in the arachnoid by R. Bright in 1831 [1], are common and have been identified more frequently due to the increasing and widespread use of computer-assisted tomography scans (CT) or magnetic resonance imaging (MRI) in recent years.
Although these cysts are usually benign and do not require aggressive treatment, some patients suffer from severe symptoms caused by the mass effect of the cyst, requiring surgical intervention.
Different causes of water retention mechanisms [2] have been described in the past, including congenital factors leading to water retention and physical factors resulting in the development of water retention.
We examined 3 cases of arachnoid cysts that occurred in different locations of the brain among different age groups of the adult population. Surgical specimens were obtained and immunohistochemical staining was performed to determine whether aquaporin has any relationship with the formation of water-containing cavities.
Case Reports
CASE 1: A 52-YEAR-OLD MAN:
The patient presented to our hospital with chief concerns of a “heavy sensation” in the head and dysesthesia for 2 months on the 2nd, 3rd, and 4th fingers of the left hand. MRI (Figure 1A, 1B) revealed a mass located in the frontal interhemispheric fissure extending to the left frontal convex. The patient was diagnosed with a similar fluid mass 5 years prior to his visit, but with a much smaller size. The large mass showed a marked space-occupying effect, which led us to perform surgical intervention.
After the dural opening following the initial left frontal craniotomy, the cavity was proved to be located mainly in the inter-hemispheric fissure (Figure 1C). The wall was minimally vascularized, containing clear and watery fluid. The cavity seemed to be created within the intra-arachnoid layer (Figure 1D). The wall was thick around the cortical bridging veins. The cavity wall was mostly resected (Figure 1E) and a part of it was sent to histopathology for immunohistochemical staining of aqua-porins. His postoperative course was uneventful, and his pre-operative neurological symptoms resolved significantly. The cavity shrank in size and nearly disappeared by the 20th postoperative day. The cavity was practically invisible 30 months later (Figure 1F).
CASE 2: A 68-YEAR-OLD MAN:
The patient suddenly presented with nausea and vertigo, later vomited, and was transferred to our hospital. MRI revealed the existence of a cerebellar hemorrhage (Figure 2A). A high-intensity mass-like lesion was also observed mainly in the left cerebellopontine angle extending to the dorsal side of the cerebellum. Conservative treatment was effective in relieving neurological symptoms. He quickly regained ambulatory function with a slight degree of cerebellar sign, which presented prominently on the right side. The persistence of cerebellar signs led us to perform a partial excision of the cavity wall (Figure 2B) 13 months after the initial event. The suboccipital dura mater was opened, then a thin, fairly vascularized cavity wall was observed (Figure 2C). The wall on the side of the cerebellar tentorium was partially thickened (Figure 2D). The dorsal side of the cavity wall and the one around the upper pons near the petrosal vein on the surface of the tentorium were excised (Figure 2E) for staining. In the postoperatively phase, the cavity showed little change in size but the patient was relieved of his ataxic symptoms (Figure 2F).
CASE 3: A 26-YEAR-OLD WOMAN:
The patient had been experiencing intermittent headaches for 10 years, which progressed to a consistent headache that led her to visit our hospital. No other neurological deficits were noted. MRI revealed a large mass lesion on the right temporal pole (Figure 3A). The signal intensity of the mass was similar to water (Figure 3B). Partial removal of the cavity wall was performed. The cavity wall was scarce in vasculature and the fluid consistency was watery (Figure 3C, 3D). The cavity was created within the arachnoid layer (Figure 3E). Her postoperative course was uneventful. Excision resulted in reduction of the mass size (Figure 3F) and complete relief of the continuous headache.
Obtained specimens were embedded with paraffin and sent for routine H-E staining and immunohistochemical staining.
Parts of the specimens were also fixed with glutaraldehyde for electron microscopic examination.
Antibodies used were highly specific rabbit antibodies to aqua-porins 1, 2, 3, 4, 5, and 9 (Alpha Diagnostic International, Inc., San Antonio, Texas, USA).
Light microscopic findings were essentially similar in all 3 cases. Cells with small nuclei showed no atypicality and were arranged like epithelia, forming several or thickened lamellae (Figure 4A). In the thick part of the cavity wall, vacuole-like spaces were observed (Figure 4B). Connective tissue was seen in between (Figure 4C). Vascularity was rarely present.
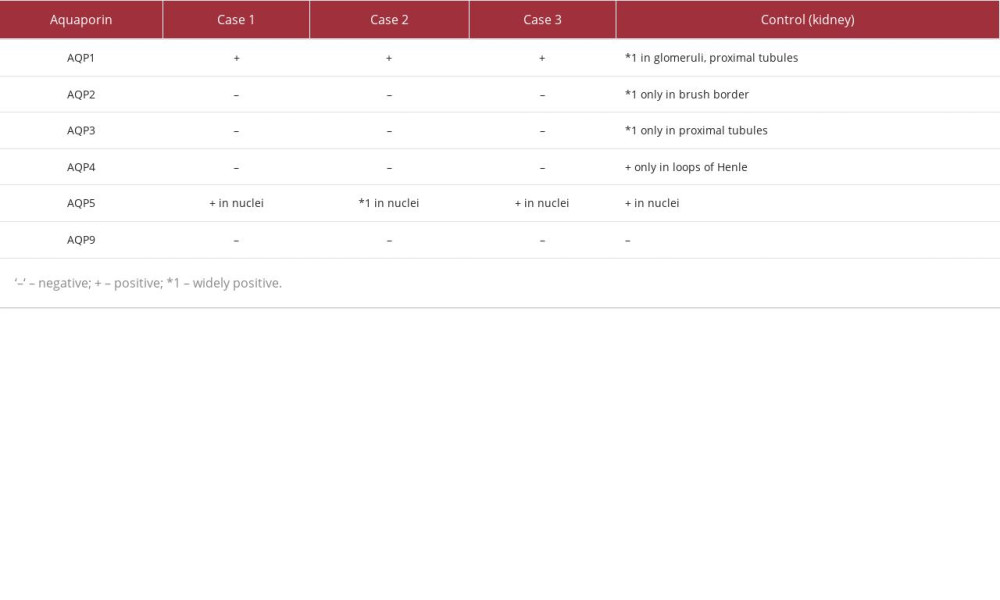
Out of the 3 cases we examined, all specimens stained globally positive for aquaporin 1 (Figure 5A). However, aquaporins 2, 3, 4, and 9 stained negative in all 3 cases. Nuclei in all 3 cases stained positive for aquaporin 5 (Figure 5B).
For comparison, kidney specimens were used, with the following findings. The glomeruli and proximal tubules widely stained positive for aquaporin 1 (Figure 5C). Only the brush border was positive for aquaporin 2 (Figure 5D). Only proximal tubules were positive for aquaporin 3 (Figure 5E). The loops of Henle were partly positive for aquaporin 4. Nuclei of the proximal tubules were positive for aquaporin 5 (Figure 5F), and no areas stained positive for aquaporin 9. Results of the aquaporin staining are summarized in Table 1.
Electron microscopy showed that cells with many or few cell organelles and with spindle-like nuclei were arranged in lamellar or flattened structures (Figure 6A). Many vacuolizations (oval colored in yellow) were seen in between. Interdigitation of cells and many desmosomes (oval colored in red) were observed (Figure 6B).
Discussion
Classically, arachnoid cysts were detected after patients presented with symptoms caused by their mass effect, but recent rise in detection due to the development of radiographic modalities such as CT and MRI have led to arachnoid cysts being found in subclinical stages. Even with the growing incidence of arachnoid cysts, its pathogenesis still seems to be ambiguous.
Some postulated that the size of arachnoid cysts seemed to be stable in adult patients [3], and expanding arachnoid cysts were seen mainly in the pediatric population [4].
Microscopic findings with H-E staining suggested water accumulation within the arachnoid layer, initiated by the splitting, tearing, or necrosis of the arachnoid layer. This phenomenon has been widely accepted since Rengachary and Watanabe analyzed over 200 cases of arachnoid cyst [5]. The initial damaged area may heal through the generation of collagen, and consequential trapping of water via a check valve mechanism may occur.
The ultrastructure of the cyst wall was similar to that of the normal arachnoid. The wall cells showed an arachnoidal formation with regions of heterogenous thickness, with varying amounts of cell organelles due to mechanical stretching and compression. A great number of small vacuolizations were also observed. These small vacuoles, with thin and fragile walls, may gradually fuse with one another to become a larger water-containing cavity via scarring, finally resulting in a large water-containing cavity.
The existence of vasogenic edema as a cause of intercellular water retention was dismissed due to the presence of firm desmosomes on the cavity wall. Firm adhesions between cells may well be the mechanism behind “non-communicating” cysts, which are completely isolated from the physiological cerebrospinal fluid (CSF) circulatory system.
Considering the mechanism of expansion of arachnoid cysts, several valve-like mechanisms have been proposed [6]. Since the arachnoid wall is thin and fragile enough, the impact force might cause the tearing or splitting on the arachnoid structure itself. Then, normal pulsatile movements of the CSF help to create a one-way valve, commonly known as a check valve, resulting in water accumulation, creating a solitary cavity. Moreover, the thin and fragile membrane of the arachnoid cyst is prone to spontaneous rupture, which may result in the size reduction.
Comparisons between congenital and acquired arachnoid cysts have also been a focus, where the osmotic gradient between cystic content and CSF seems to aid in its development [7]. However, this theory is not widely supported, since the fluid contents such as electrolytes, sugar, and urea nitrogen do not differ between the cavity fluid and the normal CSF.
Although the evaluation of microscopic structures may suggest the mechanism of the initial formation of the cavity wall, this alone does not sufficiently explain the widely varying clinical course of arachnoid cysts. While some patients were asymptomatic for a long time, other patients presented with neurological deficits that required surgical intervention. Go et al suggested the possibility of the secretory ability of the cyst cell [8]; they postulated that ATPase and alkaline phosphatase were partly responsible for the cyst cells. Rabiei et al also reported that there is a possibility of secretion because the cyst cells have rich microvilli on the luminal side of the wall in some cases [9]. However, our previously-mentioned microscopic examinations revealed that the cavity wall was composed of thin meningothelial cells with small nuclei, and contained a fair amount of connective tissue. It also stained positive for vimentin, but stained negative for EMA, CEA, D2-40, and MIB-1.
Our findings suggest that the tissues are not of epithelial or lymphatic origin, as secretion hardly occurs toward the cavity lumen. Poor vascularity in the cavity wall also indicated that secretion from the cavity wall was less active, which adds valuable evidence to our hypothesis that physiological secretion is unlikely to be a cause of simple water retention in arachnoid cysts. Additionally, fluid secretion is commonly associated with protein exudation to a certain degree, which is not consistent with our MRI findings.
Aquaporin [10], a passive, gradient-based water-transporting membrane protein, is known to have a major role in carrying water across the cell membrane [11]. Varieties of aquaporin have been reported in the central nervous system; aquaporin 1 has been typically observed in the choroid plexus, whereas aquaporin 4 and 9 are found in astrocytes, associated with its water influx. On the other hand, aquaporin 2 is seen in the brush border of the kidney, aquaporin 3 occurs in epithelial cells, and aqua-porin 5 is found in the excretory epithelia and cell nuclei [12].
Basaldella et al stated there was no evidence that aquaporin 1 was expressed in the normal arachnoid layer, arachnoid villi, and arachnoid cyst (cavity) wall [7], but our studies suggest the presence of aquaporin 1 in cavity wall cells. Specimens from all 3 cases stained positive for aquaporin 1, suggesting that fluid retention is related to the mechanism of CSF production [13], despite the poor vascularity of the cyst wall. The area that stained positive for aquaporin 1 was not equally distributed on the cavity wall. We suspect that regions with viable cell membranes stained positive for aquaporin 1, whereas the regions with weak or negative stains were dominated by scar tissue and/or connective tissue. This partial aquaporin 1 expression may explain the variable clinical presentations, together with the microscopic characteristics of the cavity wall. The existence of aquaporin expression may also explain the nature of the cavity content, which is watery fluid. Methods to quantify the function of aquaporin 1 in the arachnoid cyst wall are needed for a more precise evaluation.
In all of our cases, the cavity content was macroscopically colorless and transparent, resembling CSF, and the radiographic appearance was similar to the CSF. The cavity showed no signs of microscopic communication with the physiological CSF circulatory system. The fact that the cavity membranes stained positive for aquaporin 1, which indicates that water retention occurs even with scarce vascularity and lack of secretory structures in the cavity walls. We then hypothesized that arachnoid cysts are a secondary lesion following the fusion of numerous microvacuoles. Once they have fused and enlarged, water continues to accumulate via transportation with aquaporin over the cavity wall (Figure 7).
This hypothesis does not conflict with the change in size of the cavity. As time progresses, the gradual increase in size ceases, and the cavity can rupture into the subarachnoid space and then into the CSF circulation.
This study, with its interesting and persuasive findings, is limited by its small sample size, but has a potential role in identifying the function of aquaporin. To provide sufficient statistical evidence of involvement in its continuous enlargement, studies with larger numbers of surgical specimens from arachnoid cysts are needed for aquaporin staining.
Conclusions
Our study showed that water transportation through aquaporin 1 may be able to form and expand arachnoid cysts. While our hypothesis may contradict some studies, we believe it reinforces a number of supporting hypotheses for water retention of arachnoid cysts. The combined role of passive water flow and microtears may explain the gradual increase and decrease in cyst size.
Figures
References:
1.. Bright R, Serous cyst in the arachnoid: Reports of medical cases, selected with a view of illustrating the symptoms and cure of diseases by a reference to morbid anatomy, 1831; 437-39, London, Longman
2.. Adeeb N, Deep A, Griessenauer CJ, The intracranial arachnoid mater: A comprehensive review of its history, anatomy, imaging, and pathology: Child’s Nerv Syst, 2013; 29; 17-33
3.. Al-Holou WN, Terman S, Kilburg C, Prevalence and natural history of arachnoid cysts in adults: J Neurosurg, 2013; 118; 222-31
4.. Gosalakkal JA, Intracranial arachnoid cysts in children: A review of pathogenesis, clinical features, and management: Pediatr Neurol, 2002; 26; 93-98
5.. Rengachary SS, Watanabe I, Ultrastructure and pathogenesis of intracranial arachnoid cysts: J Neuropathol Exp Neurol, 1981; 40; 61-83
6.. Santamarta D, Aguas J, Ferrer E, The natural history of arachnoid cysts: Endoscopic and cine-mode MRI evidence of a slit-valve mechanism: Minim Invasive Neurosurg, 1995; 38; 133-37
7.. Basaldella L, Orvieto E, Dei Tos AP, Causes of arachnoid cyst development and expansion: Neurosurg Focus, 2007; 22; 1-4
8.. Go KG, Houthoff HJ, Blaauw EH, Arachnoid cysts of the sylvian fissure. Evidence of fluid secretion.: J Neurosurg, 1984; 60; 803-13
9.. Rabiei K, Tisell M, Wikkelsø C, Diverse arachnoid cyst morphology indicates different pathophysiological origins: Fluids Barriers CNS, 2014; 11; 5
10.. Agre P, The aquaporin water channels: Proc Am Thorac Soc, 2006; 3; 5-13
11.. Verkman AS, Mitra AK, Structure and function of aquaporin water channels: Am J Physiol Renal Physiol, 2000; 278; F13-28
12.. Day RE, Kitchen P, Owen DS, Human aquaporins: Regulators of transcellular water flow: Biochim Biophys Acta, 2014; 1840; 1492-506
13.. Kurokawa Y, Sohma T, Tsuchita H, A case of intraventricular arachnoid cyst. How should it be treated? Child’s: Nerv Syst, 1990; 6; 365-67
Figures
In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250