09 October 2023: Articles 
Unusual Presentation of an Uncommon Malignancy: A 74-Year-Old Woman with Aggressive Fibromatosis of the Large Intestine Presenting as a Liver Mass and the Therapeutic Management
Challenging differential diagnosis, Diagnostic / therapeutic accidents, Unusual setting of medical care, Rare disease
Christos Vallilas1ABDEF*, Stavros P. PapadakosDOI: 10.12659/AJCR.939862
Am J Case Rep 2023; 24:e939862
Abstract
BACKGROUND: Desmoid tumors are a fibroblastic proliferation of soft tissues, with an extreme inclination for local dissemination and recurrence. Surgical excision is the usual treatment choice, with data regarding pharmaceutical treatment being scarce.
CASE REPORT: A 74-year-old female patient was admitted to “Laikon” General Hospital of Athens, Greece presenting with acute kidney injury secondary to diarrhea. The ultrasound, CT, and abdominal MRI performed showed a 12×6×10 cm tumorous liver lesion. Biopsy of the lesion revealed loosely organized, mesenchymal tissue with spindle cells, and myxoid stroma. Immunochemistry was positive for SMA and b-catenin. Right hemicolectomy was performed with tumor-free surgical margins (R0 resection) and tamoxifen was initiated. Six months after the last MRI (3 months after the use of tamoxifen), a follow-up MRI was performed. The tumor had increased to 14.2×11×12.3 cm, and at the next follow-up it had grown to 20.3×19 cm maximal dimensions; no new metastases were found. The patient received sorafenib and pazopanib. Our patient had PFS with sorafenib for more than 2 years and remained in a good performance status (ECOG 1). For Pazopanid, the median PFS for this treatment option was 6.5 months.
CONCLUSIONS: The results were good and show a promising method for the treatment of this rare but severe malignancy.
Keywords: Fibromatosis, Aggressive, Pazopanib, sorafenib, Female, Humans, Aged, tamoxifen, Liver Neoplasms
Background
Desmoid tumors, also known as aggressive fibromatosis, are a fibroblastic proliferation with an extreme inclination for local dissemination and recurrence but with a limited metastasizing capacity [1]. Aggressive fibromatosis is rare, with an estimated incidence of 2–5 patients per million population annually in Europe [2], peaking at ages 25–35 years [3]. Desmoid tumors can arise in any part of the body and are generally classified in anatomic terms as extra-abdominal, abdominal, or intra-abdominal [3]. The most frequently affected areas are the extremities, the abdominal wall, and the mesentery [3]. About 85–90% of cases show mutations in the
Case Report
A 74-year-old female patient with a past medical history of arterial hypertension, hypothyroidism, generalized anxiety disorder, allergic urticaria, and cholecystectomy was admitted to “Laikon” General Hospital of Athens, Greece due to pre-renal acute kidney injury. The patient described multiple, daily episodes of watery diarrhea, with no blood or mucus, in the previous 12 days. She reported no fever, weight loss, night sweats, or abdominal pain.
On clinical evaluation, the patient was afebrile with a blood pressure of 95/60 mmHg, a heart rate of 80 beats per minute, an oxygen saturation of 98% on ambient air, and a respiratory rate of 22 breaths per minute. Stool culture isolated
Abdominal ultrasonography was performed, which revealed 2 cystic liver lesions with a maximum diameter of 4.4 cm and 3.3 cm, respectively, and a solid echogenic mass of 4.9×3.5×4.3 cm in the anatomic region of the remnant of the cystic duct, with autonomous vascularization. The abdominal computed tomography (CT), performed with only per os contrast due to sustained renal insufficiency, revealed areas of hypodensity in the liver parenchyma without any pathological findings from the pancreas, spleen, kidneys, or adrenal glands. Diverticulosis with no signs of inflammation was evident in the recto-sigmoid area. Colonoscopy revealed no lesions in the large intestine and the terminal ileus; biopsies taken showed mucosal edema and a mild increase in the chronic inflammatory constituents.
A following-up EGD revealed improvement, with areas of slight erythema in the volvulus and the second part of the duodenum. An abdominal CT with intravenous contrast was performed, showing once again the aforementioned areas of hypodensity in the liver parenchyma; an area of hypodensity with inflammatory characteristics adjacent to the inferior liver surface was also revealed. An abdominal MRI was then performed (Figure 1), which showed a 12×6×10 cm intra-abdominal tumorous lesion with irregular signal distribution, emerging from the muscularis propia of the large intestine, showing a high signal in T2 sequence, and a low signal in T1, but no marked deterioration in the diffusion sequence. The lesion seemed to be in close proximity to the right colic flexure and the proximal part of the transverse colon, causing blurring of the pericolic fat and lymph node enlargement. It is a large-intestine tumor that infiltrates the surrounding structures and also infiltrates the liver. A biopsy of the lesion was performed, showing loosely organized mesenchymal tissue with spindle cells and mucus stroma.
Immunochemistry performed in the tissue was positive for SMA and b-catenin and negative for DOG1, c-kit, CD34, S100, desmin, and MDM-2. The case was referred to the hospital’s Multidisciplinary Team Meeting (MDT) where surgical resection of the tumor was decided [6]. A right hemicolectomy was performed with tumor-free surgical margins (R0 resection). A whitish tumor that was scirrhous and elastic in palpation with dimensions of 18×7×6 cm and 10 lymph nodes of 0.1 to 0.7 cm in diameter were also resected. According to the microscopic examination, the specimen revealed a hypocellular mesenchymal neoplasm comprising spindle and oval-shaped cells with patternless arrangement in a thick collagenous background that showed areas of edema and hyalinization. Cellular atypia was minimal with a small number of mitoses observed. The tumor was accompanied by a rich inflammatory infiltrate that included plasma cells and mast cells, while necrotic areas were absent. The neoplasm invaded part of the muscularis propria extending to the pericolic fat tissues., tumor cells expressed only SMA and b-catenin on immunohistochemistry and were negative for desmin, S100, CD117, and DOG-1; thus, a diagnosis of desmoid fibromatosis was set (Figure 2).
The tissue testing of exon 18 of the
After 6 months, the patient presented with abdominal distension. Abdominal ultrasound revealed large-scale ascites and paracentesis. The ascetic fluid was transparent yellow with a total protein of 5.9 g/dL and serum-ascites albumin gradient of less than 1.1 g/dL, compatible with non-portal hypertension-induced ascites due to peritoneal cavity fibromatosis. An abdominal MRI documented the existence of a tumorous lesion arising from the fourth and fifth hepatic lobes, with a maximal dimension of 9.2×9.5 cm, adjacent to bowel loops and the right abdominal wall. First-line treatment with NSAIDs (nimesulide) and tamoxifen at 100 mgr daily was initiated [7–9]. Six months after the last MRI and 3 months after the initiation of tamoxifen, a follow-up MRI was performed [10]. The tumor had increased to 14.2×11×12.3 cm and peritoneal implantations were noted; no distant metastases in the lung or brain were found. Analogously, at the next follow-up, the tumor had reached 20.3×19 cm maximal dimensions, and no new metastases were found.
One month later, eosinophilia, with an absolute count of 4.310 cells per ml, was found in routine laboratory testing and the patient was hospitalized for investigation. Hypereosinophilia was considered a secondary reaction to her primary malignancy [11,12].
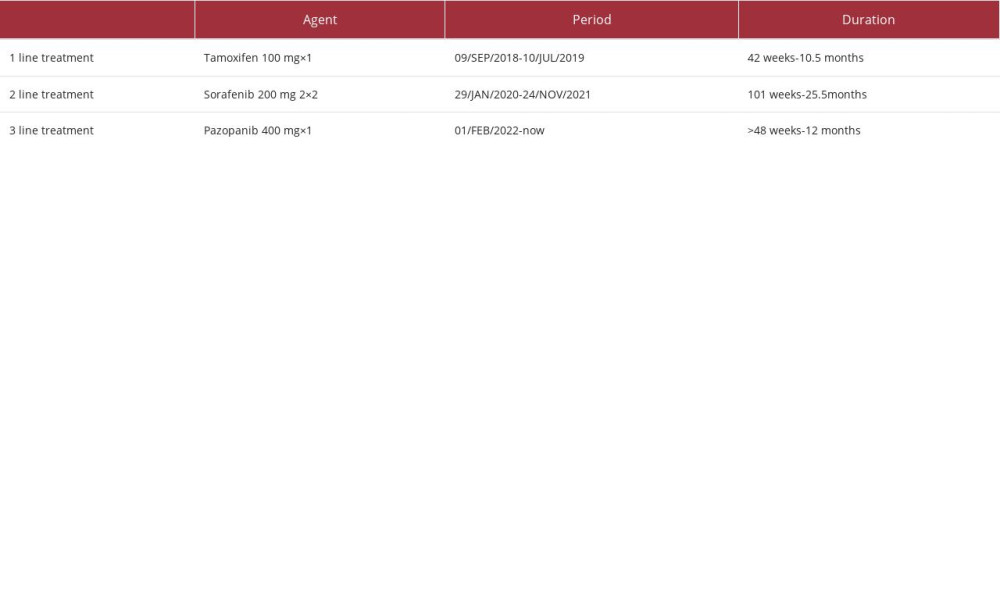
At the next follow-up 1 month later, disease progression was noticed and treatment with sorafenib 400 mg twice daily was initiated [13,14]. The patient responded well to the medical treatment, with no adverse events while she continued to receive medical care regularly for paracentesis of ascitic fluid. Disease progression was documented 21 months later, and pazopanib, a multi-targeted tyrosine kinase inhibitor (TKI), as the third line treatment was initiated at 400 mg once daily [15,16] (Table 1). Follow-up at 3 and 9 months with CT scans revealed stable disease, with a good performance status (ECOG: 1) and no serious adverse events.
Discussion
Desmoid tumors exhibit variable clinical outcomes. The primary treatment approach was surgery but there has been a recent shift toward a more conservative management strategy with the aim of standardizing practices among clinicians [17]. The initial approach of “active surveillance” is considered as the first step after diagnosis for most patients [18]. Due to the variable nature of the disease, other treatments should only be given in cases of persistent progression [19]. “Active surveillance” involves continuous monitoring of patients, typically with an initial MRI (or CT if MRI is not feasible) within the first 2 months, followed by assessments at 3- to 6-month intervals. Our patient had contrast-enhanced MRI at primary diagnosis and at MRI follow-up, distinguishing postoperative soft-tissue changes and recurrent tumor [20,21]. The decision to pursue active treatment should be delayed until progression is observed, confirmed by at least 2 further assessments and preferably not before 1 year from the time of diagnosis. This approach helps prevent overtreatment in patients who may experience spontaneous regression. A quicker decision to pursue active therapy may be made if the disease is located near a critical structure that could significantly impact the patient’s quality of life [17,22].
In cases of progression of abdominal wall desmoid tumors, surgery continues to be the primary treatment choice [23]. However, for desmoid tumors located in the intra-abdominal/ retroperitoneal/pelvic regions, systemic therapy should be considered as the initial treatment option. For desmoid tumors in the extremities, pelvis, or chest wall, surgery should not be the first-line treatment unless the anticipated negative impact on the patient’s well-being is minimal. In such instances, the decision should be made after consulting a multidisciplinary team (MDT) [17,24]. For desmoid tumors in the head and neck or intrathoracic region, medical treatment is commonly considered as the first-line option [17,24]. Radiotherapy can be a reasonable and effective alternative as the first-line treatment in specific situations (eg, older age, patient intolerance/preference, comorbidities, rapid tumor growth near vital organs) [17].
Desmoid tumors associated with familial adenomatous polyposis (FAP) or Gardner syndrome tend to be more aggressive and multifocal, requiring more aggressive medical management [25]. Biopsies should be approached with caution, although currently there is insufficient data to completely rule out their use.
Regarding the choice of medical therapy, the lack of comparative studies prevents the proposal of a specific treatment algorithm [21]. Currently, randomized data are available only for sorafenib, pazopanib, and methotrexate plus vinblastine [17,24,26]. Prospective phase II studies have been undertaken to evaluate the efficacy of administering low-dose chemotherapy combining methotrexate and vinblastine, as well as use of imatinib [26,27]. Generally, it is advisable to initiate treatment with less toxic therapies and progress to more toxic agents in a stepwise manner [17,28]. When selecting a systemic treatment option from the various possibilities, several factors should be considered, including [17] the level of evidence, [18] overall response rate, [19] progression-free survival (PFS) rate, [22] ease of administration, and [23] expected drug toxicity [17].
Conclusions
In conclusion, intra-abdominal desmoid tumors are rare, and there are few clinical trials, case reports, and data in the literature. The therapeutic strategies combine surgical intervention and chemotherapy. Our patient received the newest therapies for desmoids tumors and is in a very good performance status without any serious adverse events. The results so far are good, proving this treatment’s efficacy and revealing a promising method for treatment of this rare but severe malignancy.
Figures
References:
1.. Riedel RF, Agulnik M, Evolving strategies for management of desmoid tumor: Cancer, 2022; 128; 3027-40
2.. Penel N, Coindre JM, Bonvalot S, Management of desmoid tumours: A nationwide survey of labelled reference centre networks in France: Eur J Cancer, 2016; 58; 90-96
3.. Constantinidou A, Scurr M, Judson I, Litchman C, Clinical presentation of desmoid tumors: Desmoid Tumors, 2012; 5-16
4.. Kasper B, Baumgarten C, Garcia J, An update on the management of sporadic desmoid-type fibromatosis: A European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG): Ann Oncol, 2017; 28; 2399-408
5.. Garcia-Ortega DY, Martín-Tellez KS, Cuellar-Hubbe M, Desmoid-type fibromatosis: Cancers (Basel), 2020; 12(7); 1851
6.. von Mehren M, Randall RL, Benjamin RS, Soft tissue sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology: J Natl Compr Canc Netw, 2016; 14(6); 758-86
7.. Hansmann A, Adolph C, Vogel T, High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors: Cancer, 2004; 100(3); 612-20
8.. Janinis J, Patriki M, Vini L, The pharmacological treatment of aggressive fibromatosis: A systematic review: Ann Oncol, 2003; 14(2); 181-90
9.. de Camargo VP, Keohan ML, D’Adamo DR, Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor): Cancer, 2010; 116(9); 2258-65
10.. Gondim Teixeira PA, Chanson A, Verhaeghe JL, Correlation between tumor growth and hormonal therapy with MR signal characteristics of desmoid-type fibromatosis: A preliminary study: Diagn Interv Imaging, 2019; 100(1); 47-55
11.. Guo C, Bochner BS, Workup for eosinophilia: Allergy Asthma Proc, 2019; 40(6); 429-32
12.. Wardlaw AJ, Wharin S, Aung H, The causes of a peripheral blood eosinophilia in a secondary care setting: Clin Exp Allergy, 2021; 51(7); 902-14
13.. Benech N, Walter T, Saurin JC, Desmoid tumors and celecoxib with sorafenib: N Engl J Med, 2017; 376(26); 2595-97
14.. Gounder MM, Mahoney MR, Van Tine BA, Sorafenib for advanced and refractory desmoid tumors: N Engl J Med, 2018; 379(25); 2417-28
15.. Agresta L, Kim H, Turpin BK, Pazopanib therapy for desmoid tumors in adolescent and young adult patients: Pediatr Blood Cancer, 2018; 65(6); e26968
16.. Toulmonde M, Pulido M, Ray-Coquard I, Pazopanib or methotrexatevinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): A non-comparative, randomised, open-label, multicentre, phase 2 study: Lancet Oncol, 2019; 20(9); 1263-72
17.. Alman B, Attia S, Baumgarten C, The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients: Eur J Cancer, 2020; 127; 96-107
18.. Kasper B, Baumgarten C, Garcia J, An update on the management of sporadic desmoid-type fibromatosis: A European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG): Ann Oncol, 2017; 28; 2399-408
19.. Kasper B, Ströbel P, Hohenberger P, Desmoid tumors: Clinical features and treatment options for advanced disease: Oncologist, 2011; 16; 682-93
20.. Sedaghat S, Sedaghat M, Krohn S, Long-term diagnostic value of MRI in detecting recurrent aggressive fibromatosis at two multidisciplinary sarcoma centers: Eur J Radiol, 2021; 134; 109406
21.. Sedaghat S, Surov A, Krohn S, Configuration of primary and recurrent aggressive fibromatosis on contrast-enhanced MRI with an evaluation of potential risk factors for recurrences in MRI follow-up: Rofo, 2020; 192(5); 448-57
22.. Kasper B, Gruenwald V, Reichardt P, Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG): Eur J Cancer, 2017; 76; 60-67
23.. Cates JMM, Prognostic factors for second recurrence after surgical resection of recurrent desmoid-type fibromatosis: Pathol Oncol Res, 2015; 21; 1085-90
24.. Duggal A, Dickinson IC, Sommerville S, Gallie P, The management of extra-abdominal desmoid tumours: Int Orthop, 2004; 28; 252-56
25.. Gega M, Yanagi H, Yoshikawa R, Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis: J Clin Oncol, 2006; 24; 102-5
26.. Palassini E, Frezza AM, Mariani L, Long-term efficacy of methotrexate plus vinblastine/vinorelbine in a large series of patients affected by desmoid-type fibromatosis: Cancer J, 2017; 23; 86-91
27.. Chugh R, Wathen JK, Patel SR, Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial: Clin Cancer Res, 2010; 16; 4884-91
28.. Zhang Z, Shi J, Yang T, Management of aggressive fibromatosis: Oncol Lett, 2021; 21; 43
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250