04 September 2023: Articles 
Gastric and Duodenal Fistulas in Crohn’s Disease, a Surgical Challenge: Report of 5 Cases and a Review of the Literature
Challenging differential diagnosis, Rare disease, Educational Purpose (only if useful for a systematic review or synthesis)
Carlos Walter Sobrado JuniorDOI: 10.12659/AJCR.940644
Am J Case Rep 2023; 24:e940644
Abstract
BACKGROUND: Fistulas involving the stomach and duodenum in Crohn’s disease are rare (occurring in less than 1% of patients). Here, we reviewed registers from 855 patients with Crohn’s disease treated in our service from January 2007 to December 2020 and found 4 cases of duodenal fistula and 1 case of gastric fistula.
CASE REPORT: The fistula origin was in the ileocolic segment in all cases, and all of the patients underwent preoperative optimization with improvement of nutritional status and infection control. They then underwent surgical treatment with resection of the affected segment and duodenal or gastric closure with covering by an omental patch. One case of a duodenal fistula was complicated by duodenal dehiscence. This was treated surgically with duodenojejunostomy. Each of the other patients had an uneventful postoperative course. All patients were successfully cured of their gastroduodenal fistulas, and at the time of this publication, none of them died or had fistula recurrence.
CONCLUSIONS: Fistulas with the involvement of the stomach and duodenum in patients with Crohn’s disease are almost always due to inflammation in the ileum, colon, or previous ileocolic anastomosis. Management of this situation is complex and often requires clinical and surgical assistance; preoperative optimization of the patient’s general condition can improve the surgical results. The surgical approach is based on resection of the affected segment and gastric or duodenal closure with covering by an omental patch. Gastrojejunostomy or duodenojejunostomy can be performed in selected patients with larger defects and minor jejunal disease. To prevent recurrence, prophylactic therapy with anti-TNF agents and early endoscopic surveillance are also essential for successful treatment.
Keywords: Crohn Disease, Duodenum, Fistula, Stomach, Humans, Tumor Necrosis Factor Inhibitors, Intestinal Fistula
Background
Fistulas are a common complication of Crohn’s disease (CD), but a fistula with involvement of the stomach and duodenum is a rare condition affecting approximately 0.3–5% of CD patients [1]. This condition usually results from inflammation in other sites of the gastrointestinal tract. Management of these cases is complex and involves preoperative optimization and surgical treatment; however, there is controversy regarding the optimal surgical approach, which may include primary duodenal closure or more complex procedures such as duodenojejunostomy [2–5]. Postoperative maintenance therapy with anti-TNF agents is important to prevent recurrence, a topic that is not well discussed in published studies about this specific condition. Such controversies are justified by actual evidence that is based on case reports and case series. At the same time, larger studies clarifying the optimum pre-operative preparation, the ideal surgical procedure, and long-term maintenance therapy and outcomes are lacking. Here, we reviewed 855 medical records of CD patients treated in our service and found 4 cases of duodenal fistula and 1 case of gastric fistula. We describe each case and discuss aspects of management of this condition.
Case Reports
CASE 1:
A 70-year-old man with a 4-year history of CD presented with abdominal pain, nausea, diarrhea, and weight loss. Computed tomography (CT) enterography demonstrated thickening of the ileal wall, a transverse-ileal fistula, and an ileum-duodenal fistula (Figure 1). He underwent typhlectomy with primary ileocolic anastomosis and primary duodenal closure in 2 layers of transversal suture associated with an omental patch.
CASE 2:
A 65-year-old man who had been followed for CD for 18 years presented with abdominal pain, fever, and diarrhea. CT demonstrated pancolitis, and colonoscopy revealed stenosis of the sigmoid colon. He underwent an exploratory laparotomy with additional findings of fistula involving the transverse colon and third duodenal portion. A subtotal colectomy was performed, and a transverse section of the duodenum was covered with an omental patch using a linear stapler.
CASE 3:
A 33-year-old man had been followed for CD for 18 years. He had a past medical history of typhlectomy 4 years previously, and presented with abdominal pain, weight loss, and cutaneous fistulas. CT revealed an ileocolic-duodenal fistula and fistulous paths to the right psoas and right inferior quadrant skin. Upper digestive endoscopy (UDE) demonstrated a suspected duodenal fistula (Figure 2). He underwent ileocolonic anastomosis resection with terminal ileostomy and mucosal fistula of the transverse colon along with resection of cutaneous fistulas and primary duodenal closure, which was covered with an omental patch.
CASE 4:
A 47-year-old man, with an 8-year history of CD and a medical history of right ileocolectomy 5 years previously, presented with abdominal pain, fecaloid vomiting, and weight loss. CT demonstrated thickening of the ileotransverse anastomosis and fistula to the second duodenal portion. He underwent anastomosis resection, duodenal closure, and an omental patch. A presentation of postoperative duodenal dehiscence was treated surgically with duodenojejunostomy.
CASE 5:
A 47-year-old woman with CD that had been monitored for 5 years presented to our service with diarrhea, thin stools, weight loss, and fecaloid vomiting. CT demonstrated strictures involving the transverse and descending colon and a cologastric fistula involving the splenic flexure (Figure 3). UDE showed a cologastric fistula, and a nasoenteric catheter was placed. She underwent extended left colectomy and primary reconstruction as well as gastric fistula debridement with primary closure in 2 layers.
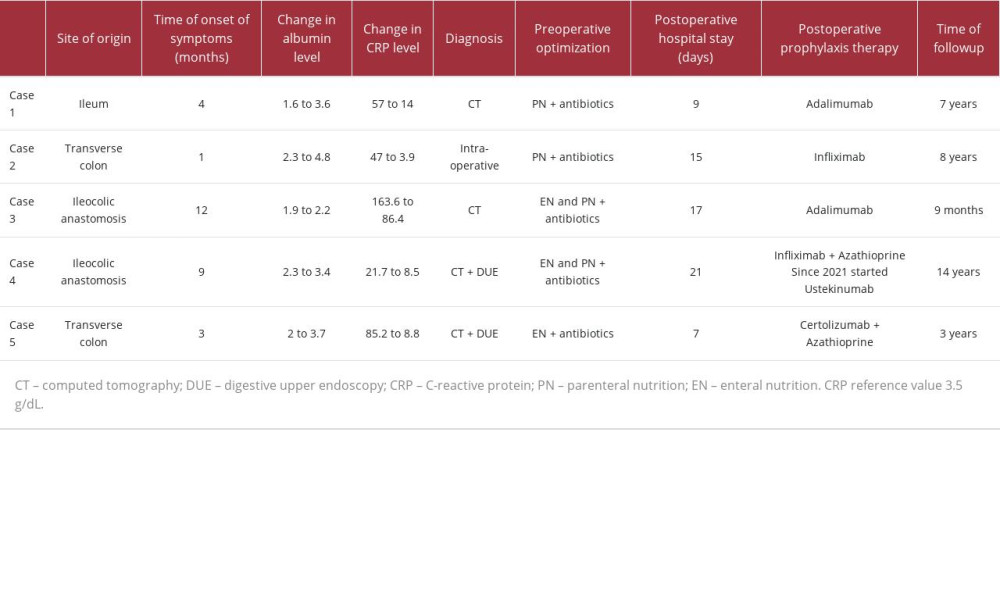
All patients had an impaired nutritional status and all of them underwent preoperative optimization with nutritional therapy and antibiotics. All patients received postoperative maintenance therapy with anti-TNF agents to prevent recurrence and were followed up closely with early endoscopic surveillance in the first year after resection. Except for the complications related to Case 4, there were no other complications in the short- or long-term followup. Details regarding each case are presented in Table 1.
Discussion
Gastric and duodenal fistulas are rare complications of CD and usually result from disease activity in the ileum, ascending and transverse colon, or ileocolic anastomosis. Symptoms are usually nonspecific, such as diarrhea and abdominal pain, but more specific symptoms can occur, including fecaloid vomiting and intestinal malabsorption, when long bowel segments are bypassed. Fistulas originating from intrinsic duodenal CD are extremely rare [2,3,5]. Klein described a prevalence of 0.5% of duodenal fistulas in CD patients [6]. Michelassi reported that 5% of 290 CD fistulas presented with gastroduodenal involvement [4]. Greenstein described gastric fistulas affecting 0.8% of patients with ileocolitis and 0.6% of patients with colitis [7]. In our study, a fistulizing pathway affecting the stomach and duodenum occurred in 0.58% of patients with CD, similar to other studies. The simultaneous infection of the stomach with
Wilk suggested that CD patients with previous ileocolic resection are at a higher risk for gastric and duodenal fistulas, and some authors argue that the omentum should be placed between the anastomosis and duodenum in ileocolectomy for CD to avoid this complication [1,5]. Gong found an increased rate of recurrence of coloduodenal fistula in patients whose fistula originated from a previous anastomosis [8].
Gastric and duodenal fistulas are not an absolute indication for surgery. A recent prospective cohort study showed that biological agents can effectively bring closure in a quarter of patients with internal fistula tracts [9].
The use of infliximab has been reported, with good results in cases of refractory duodenal ulcers, duodenal stenosis, and duodenal fistulas [10,11]. In a prospective study, Annunziata et al [12] demonstrated that among 19 patients with gastroduodenal CD, 72.7% of the patients treated with infliximab or adalimumab achieved mucosal healing 12 weeks after the initiation of treatment. Other reports [13,14] have also supported the efficacy of adalimumab treatment in closing internal fistulas.
The efficacy of ustekinumab has also been demonstrated, but evidence supporting its efficacy for enterocutaneous fistula is sparse. However, we did find 2 reports of upper enterocutaneous fistula closures that were successfully treated with ustekinumab [15,16].
Surgery is the most common treatment if biological agents are not as efficient as they are for perianal fistulas [17–19]. Resection of the involved segment and closure of duodenal defects are the basis for surgical treatment [2–4,20]. Michelassi et al [4] and Lee and Schraut [20] reported complication rates of 33.3% and 18.2% and mortality rates of 0% and 9.1% for surgical treatment of duodenal fistulas, respectively. Before a patient undergoes a laparotomy, it is very important to assess the patient’s general condition and nutritional status, as was performed in our cases, to achieve adequate preoperative optimization. This includes nutritional therapy with enteral and/or parenteral nutrition. Preoperative enteral nutrition has been shown, in a guideline review, to reduce the rate of postoperative complications in malnourished CD patients undergoing surgery [21].
When present, control of abdominal septic conditions with antibiotics and percutaneous drainage should be performed if needed, and surgical treatment should be postponed until better conditions are achieved. Preoperative abscess and low albumin levels are independent risk factors for intra-abdominal septic complications, as shown by a recent meta-analysis [22], and improvement of nutritional status and reduction of inflammation, marked by improvement of serum albumin and CPR levels, have been associated with fewer postoperative complications [23,24]. Specifically, regarding coloduodenal fistulas, Gong reported that preoperative optimization decreases major postoperative and intra-abdominal infectious complications, and also lowers the rates of temporary fecal diversion [8]. In these cases, no patient had pancreatic involvement. Two patients had increased amylase, but without symptoms or clinical diagnosis of pancreatitis.
Controversy exists about the ideal surgical procedure for duodenal closure, and some authors suggest that duodenal defects could be treated successfully in most cases with primary transverse closure followed by an omental patch when feasible [3,5,8,20] (Figure 4). Complication rates range from 18.2% for morbidity and 9.1% for mortality in an older series, as described by Lee [20]. A morbidity of 28–50% and zero mortality were reported in a recent series by Gong [8]. This is consistent with our findings. For larger defects (larger than 3 cm), and in older studies, some authors have reported good results with duodenojejunostomy (Figure 4) since there is no extensive jejunal disease, as seen in the reoperation in Case 4. Case 4 represents the first reported case in the literature using this technique as a bail-out strategy. A serosal patch with the jejunum is also an alternative [1,2,20]. The most recent and largest series reporting coloduodenal fistulas in CD was published by Gong and showed that an even larger defect could be treated with primary closure, and the duodenal defect size was not associated with a higher incidence of fistulas or stenosis. However, larger series are needed to confirm these findings [8]. Temporary duodenal decompression with a nasoduodenal catheter is an important strategy in postoperative care, and a double lumen enteral catheter can be used for simultaneous decompression and feeding [5,18,25].
Because of the rarity of duodenocolic fistula cases, the reported data in the literature with CD are limited [26].
Postoperative recurrence should be a concern after ileocolonic intestinal resection, and prophylactic treatment is recommended for patients with one or more risk factors, which include smoking, prior intestinal surgery, granulomas, myenteric plexitis in the resection specimen, and penetrating disease at the index surgery. All of our patients had at least one risk factor and were at high risk of recurrence. Thiopurines and anti-TNF agents are the drugs of choice for this purpose, and high-dose mesalazine and metronidazole can also be used. However, anti-TNF agents seem to be the most effective. At this point in time, no fixed strategy is recommended, and therapy decisions should be individualized [27,28]. Due to the lack of details and discussion regarding postoperative recurrence prophylaxis in the published reports of gastric and duodenal fistula in CD, all of our patients started receiving anti-TNF agents 3 to 6 weeks after surgery. Regardless of the prophylactic therapy indicated, close followup with clinical evaluation, biomarkers, and early endoscopic surveillance with ileocolonoscopy in the first year after surgery are essential to early recurrence detection and successful management [27–29].
A multidisciplinary approach in the management of patients with inflammatory bowel disease is essential, especially in these surgical situations, in which gastroenterologists, nutritionists, and surgeons must work together to seek the best therapy for patients with such a complex disease.
The authors clarify that our outpatient care center for patients with inflammatory bowel diseases is located at a public hospital in a city of 14 million inhabitants. Patients who are in consultation are referred by the national public health system. Patients who arrive for followup of the disease, in most cases, already present with a severe phenotype of the disease, sometimes without prior correct diagnosis and without adequate treatment. These patients also require time to be hospitalized and undergo tests.
Conclusions
Despite being a rare and complex complication of CD, gastric and duodenal fistula can be treated successfully with preoperative optimization, resection of the compromised intestinal segment followed by duodenal or gastric closure and covering by an omental patch, and adequate postoperative follow-up with endoscopic surveillance and prophylactic treatment to prevent postoperative recurrence.
Figures
References:
1.. Wilk PJ, Fazio V, Turnbull RB, The dilemma of Crohn’s disease: Dis Colon Rectum, 1977; 20; 387-92
2.. Yamamoto T, Bain IM, Connolly AB, Keighley MRB, Gastroduodenal fistulas in Crohn’s disease: Dis Colon Rectum, 1998; 41; 1287-92
3.. Poggioli G, Stocchi L, Laureti S, Duodenal involvement of Crohn’s disease: Dis Colon Rectum, 1997; 40; 179-83
4.. Michelassi F, Stella M, Balestracci T, Incidence, diagnosis, and treatment of enteric and colorectal fistulae in patients with Crohn’s disease: Ann Surg, 1993; 218; 660-66
5.. Pichney LS, Fantry GT, Graham SM, Gastrocolic and duodenocolic fistulas in Crohn’s disease: J Clin Gastroenterol, 1992; 15; 205-11
6.. Klein S, Greenstein AJ, Sachar DB, Duodenal Fistulas in Crohn’s Disease: J Clin Gastroenterol, 1987; 9; 46-49
7.. Greenstein AJ, Present DH, Sachar DB, Gastric fistulas in Crohn’s disease: Dis Colon Rectum, 1989; 32; 888-92
8.. Gong J, Wei Y, Gu L, Outcome of surgery for coloduodenal fistula in Crohn’s disease: J Gastroint Surg, 2016; 20; 976-84
9.. Barreiro-de Acosta M, Fernández-Clotet A, Mesonero F, BIOSCOPE Study Group from the ENEIDA Registry. Long-term outcomes of biological therapy in Crohn’s disease complicated with internal fistulizing disease: BIOSCOPE study from GETECCU.: Am J Gastroenterol, 2023; 118; 1036-46
10.. del Carmen Rodríguez-Grau M, Chaparro M, Díaz R, Gisbert JP, [Infliximab in the treatment of refractory gastroduodenal Crohn’s disease.]: Gastroenterol Hepatol, 2014; 37; 21-22 [in Spanish]
11.. Pajares JA, Hernández L, Menchén P, Menchén L, Duodenopancreatic fistula complicating upper gastrointestinal Crohn’s disease: Succesful treatment with infliximab: Am J Gastroenterol, 2009; 104; 1863-64
12.. Annunziata ML, Caviglia R, Papparella LG, Cicala M, Upper gastrointestinal involvement of Crohn’s disease: A prospective study on the role of upper endoscopy in the diagnostic work-up: Dig Dis Sci, 2012; 57; 1618-23
13.. Tursi A, Duodenal Crohn’s disease successfully treated with adalimumab: Endoscopy, 2011; 43; E22
14.. Gaggar S, Scott J, Thompson N, Pyloric stenosis associated Crohn’s disease responding to adalimumab therapy: World J Gastrointest Pharmacol Ther, 2012; 3; 97
15.. Madarame A, Kimura H, Kunisaki R, Successful treatment with ustekinumab for enterocutaneous fistulas in Crohn’s disease: J Crohns Colitis, 2020; 14; 569-70
16.. Li H, Xie L, Yao H, Successful non-operative treatment of enterovesical and enterocutaneous fistulas due to Crohn’s disease: Int Med Case Rep J, 2022; 15; 117-24
17.. Broe PJ, Bayless TM, Cameron JL, Crohn’s disease: Are enteroenteral fistulas an indication for surgery?: Surgery, 1982; 91; 249-53
18.. Nakagoe T, Sawai T, Tsuji T, Successful resection of a duodenal fistula complicated with recurrent Crohn’s disease at the site of previous ileocolonic anastomosis: Report of a case: Surg Today, 2003; 33; 537-41
19.. Levy C, Tremaine WJ, Management of internal fistulas in Crohn’s disease: Inflamm Bowel Dis, 2002; 8; 106-11
20.. Lee KKW, Diagnosis and treatment of duodenoenteric fistulas complicating Crohn’s disease: Arch Surg, 1989; 124; 712
21.. Lin A, Micic D, Nutrition considerations in inflammatory bowel disease: Nutr Clin Pract, 2021; 36; 298-311
22.. Huang W, Tang Y, Nong L, Sun Y, Risk factors for postoperative intra-abdominal septic complications after surgery in Crohn’s disease: A meta-analysis of observational studies: J Crohns Colitis, 2015; 9; 293-301
23.. Van Assche G, Lewis JD, Lichtenstein GR, The London position statement of the World Congress of Gastroenterology on biological therapy for IBD with the European Crohn’s and Colitis Organisation: Safety: Am J Gastroenterol, 2011; 106; 1594-602
24.. Zuo L, Li Y, Wang H, A practical predictive index for intra-abdominal septic complications after primary anastomosis for Crohn’s disease: Dis Colon Rectum, 2015; 58; 775-81
25.. Zuiki T, Meguro Y, Kumano H, Successful management of a colo-duodenal fistula in a patient with Crohn’s disease using a double lumen gastro-jejunostomy tube: Case Rep Gastroenterol, 2014; 8; 162-68
26.. Bora G, Cagdas Sonbahar B, Ozalp N, A rare complication of Crohn disease: Duodenocolic fistula: Turkish J Gastroenterol, 2017; 28; 498-501
27.. Gionchetti P, Dignass A, Danese S, 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical management and special situations: J Crohns Colitis, 2017; 11; 135-49
28.. Kotze PG, Yamamoto T, Damiao AOMC, Postoperative approach for Crohn’s disease: The right therapy to the right patient: Curr Drug Targets, 2018; 19; 729-39
29.. De Cruz P, Kamm MA, Hamilton AL, Crohn’s disease management after intestinal resection: A randomised trial: Lancet, 2015; 385; 1406-17
Figures
In Press
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942660
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943174
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943136
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943645
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250