19 July 2023: Articles 
Unusual Presentation of Pediatric Scurvy: A Necrotic Gastrostomy Tube Site in a 14-Year-Old Boy
Unusual clinical course, Challenging differential diagnosis, Rare disease
Nicholas R. ZessisDOI: 10.12659/AJCR.940770
Am J Case Rep 2023; 24:e940770
Abstract
BACKGROUND: Despite being considered a disease of the past, pediatric scurvy is increasingly reported in developed countries, especially among children with autism spectrum disorder, developmental delays, or a restrictive diet. Pediatric patients typically present with lower extremity pain or refusal to walk. This case study features an atypical presentation of scurvy in a non-ambulatory patient.
CASE REPORT: A 14-year-old boy with arthrogryposis multiplex congenita displayed a late-stage scurvy symptom: a necrotic gastrostomy tube site, indicative of poor wound healing due to vitamin C deficiency. The usual telltale symptoms of scurvy were camouflaged due to his non-ambulatory status, which may have contributed to a delayed presentation. Nevertheless, a comprehensive clinical evaluation, incorporating diet history, gingival symptoms, petechiae, and characteristic radiological signs, eventually led to the correct diagnosis. Although acute osteomyelitis was initially suspected, it was subsequently ruled out. Upon initiation of vitamin C therapy, the patient's symptoms subsided within a few days, and the necrotic tissue surrounding the gastrostomy tube healed completely within two weeks.
CONCLUSIONS: The highlighted case underscores the importance of including scurvy in the differential diagnosis for pediatric patients with lower extremity pain without fever. A detailed dietary history focusing on vitamin C intake is crucial during clinical evaluation. Early initiation of vitamin C therapy, when scurvy is suspected, may prevent unnecessary and extensive diagnostic workup for other potential causes, offering timely relief to the patient.
Keywords: Arthrogryposis Multiplex Congenita Neurogenic Type, osteomyelitis, Scurvy, Male, Humans, Child, Adolescent, Ascorbic Acid, Autism Spectrum Disorder, Gastrostomy, Pain
Background
Vitamin C (ascorbic acid) deficiency, historically known as scurvy, has seen a relative resurgence in the pediatric literature over the last decade, despite fortification of food and availability of nutritional supplements in developed countries [1]. At a large referral center in the United States there were 32 cases from 2011 to 2015 [2]. Children with autism spectrum disorder, developmental delay, or food selectivity with a restricted diet are at higher risk for nutritional deficiency [1,3–8]. The disease is rare in previously healthy and developmentally appropriate children, but a restrictive diet alone can put them at risk [2,8–10].
Vitamin C plays an important biochemical role in many organ systems [5,10,11]. Deficiency occurs after several months of inadequate intake, with symptoms typically starting within 1–3 months [5,10]. Vegetables and fruits are the best vitamin C sources [5,11–13].
Clinical manifestations of scurvy are classified as early or late. Initial mild nonspecific symptoms include irritability, loss of appetite, and fatigue [10,11]. Hematologic and dermatologic manifestations represent moderate disease and present next with petechiae, purpura, ecchymosis, epistaxis, hemarthrosis, and corkscrew hairs [1,10,11]. With defective pericapillary collagen, capillary fragility results, leading to scurvy’s hematologic findings [12]. Then, late manifestations include gingival involvement, such as hypertrophy, edema, and hemorrhage, as well as skeletal manifestations [1,3,10,11]. Impaired collagen production causes a defect in bone structure, which manifests clinically as bone pain, limp, or refusal to walk, with the lower extremities most affected in children [1,5,10,12,14]. If untreated, the most severe and advanced symptoms include poor wound healing, psychological changes, and death [10,11].
Necrosis is rare in scurvy, with the literature limited to several case reports of resolution of necrotic lesions after identifying and treating hypovitaminosis C [15,16]. The pathophysiology of necrosis in this setting is not clear, but is likely related to the role of vitamin C in tissue healing [15–17]. Ascorbic acid influences tissue repair and regeneration via the synthesis of connective tissue, especially collagen. It is an antioxidant, playing an important role in neutralizing oxidants in the epidermis [17,18]. Vitamin C also increases the proliferation of dermal fibroblasts, which promotes wound healing [17,19].
We describe a novel presentation of scurvy: a 14-year-old boy with arthrogryposis multiplex congenita and a necrotic gastrostomy tube (GT) site. We highlight the clinical pitfalls and current practice variation in the diagnosis and management of scurvy, all to propose modifications to future practice.
Case Report
A fourteen-year-old white boy with arthrogryposis multiplex congenita complicated by expressive speech delay, lower-extremity limb deformities with clubfoot and wheelchair dependence, and dysphagia with prior GT dependence (ie, unable to safely consume an appropriate caloric intake by mouth), presented to the wound clinic. His arthrogryposis multiplex congenita had been previously diagnosed by an outside institution. Ultimately, at our institution, our Genetics team was clinically concerned for the same diagnosis, even though his Arthrogryposis Genetic Panel (GeneDx, Gaithersburg, MD) was negative. A genetic diagnosis is identified in 58–65% of individuals with arthrogryposis [20,21]. The etiology of his oral motor dysfunction was unclear.
A GT was placed at age 3 months for oral motor dysfunction. Eighteen months prior to presentation, he was cleared to eat by mouth with formula supplementation via his GT, working towards the goal of GT removal. At the time of presentation, he was eating only by mouth and had not used his GT for feeding in 5 months. Surgically, he had multiple right knee orthopedic operations related to his arthrogryposis. He is non-ambulatory at baseline. Two weeks prior, he developed progressively worsening pain and bleeding around his GT site, with the tissue around his stoma turning black. He also developed a rash on his lower extremities with bilateral lower- limb pain, most notably in his right knee.
Physical exam was concerning for necrosis encircling his GT site. There was no erythema, drainage, induration, or fluctuance (Figure 1). A skin exam revealed diffuse petechiae on his bilateral lower extremities from ankle to hip. Musculoskeletal exam demonstrated pain on passive motion of his bilateral ankles, knees, and hips, with tenderness, mild edema, and warmth of the right knee. There was no edema, erythema, or tenderness to his bilateral calf muscles.
He was referred to the Emergency Department (ED), presenting with tachycardia and diffuse abdominal pain (Table 1). He received fluid resuscitation and laboratory studies (Table 1), which ruled out SARS-CoV-2, but demonstrated an elevated D-dimer and fibrinogen. Radiographs of the right knee (Figure 2) were obtained and single doses of ceftriaxone and metronidazole were administered with a concern for sepsis. Abdominal imaging was unremarkable. His tachycardia and abdominal pain resolved and he was admitted to the Surgery Service. The ED was less concerned for sepsis at the time of transfer to the floor.
On admission, Pediatric Medicine was consulted for investigation of lower-extremity petechiae. Diet history revealed a severely restricted diet, consisting of mainly prepackaged grains with no fruit, vegetable, or vitamin supplementation intake (Table 2). Further examination revealed gingival hypertrophy of the posterior maxilla.
The GT was removed; however, no surgical intervention was performed on the surrounding necrosis. Our surgical consultants felt that there would be minimal surgical benefit and recommended medical therapy first, with reconsideration for surgery if medical therapy failed.
In the setting of petechiae with unremarkable prothrombin time and activated partial thromboplastin time, assessment for von Willebrand disease was unremarkable (Table 3). On hospital day 1, magnetic resonance imaging (MRI) of the right leg was obtained, which was initially concerning for acute osteomyelitis. Although scurvy was on our differential diagnosis, we initially were more concerned for osteomyelitis given his prior lower-extremity surgical history and the MRI findings, which delayed initiation of vitamin C therapy. Cefazolin was started for empiric
He was never febrile during his admission, which prompted reevaluation of his MRI findings. The inflammatory changes were thought to be more consistent with vitamin C deficiency than acute infection (Figure 3). Intravenous vitamin C (100 mg every 8 hours for 1 week) was initiated on hospital day 3. With the intention to not miss a skeletal infection, he was continued on antibiotics until the return of a low serum vitamin C level (Table 3) on hospital day 8. Within 2 days of starting treatment for scurvy, his lower-extremity pain, petechiae, and C-reactive protein (CRP) had all improved. He was also found to be deficient in vitamin D and B6, with multivitamin supplements added to his regimen. He was discharged home on an oral vitamin C supplement of 100 mg daily and received nutrition education.
At follow-up 2 weeks after discharge (and after 17 total days of vitamin C supplementation), his former GT site was well-healed. Surgical intervention of the necrotic lesion was not ultimately necessary given his improved wound healing with vitamin C therapy [15–19]. Repeat vitamin levels were scheduled for 4 weeks after discharge, as was investigation for celiac disease, but the patient did not follow up.
Although he was at increased risk for deep vein thrombosis (DVT) given his non-ambulatory status [20,21], additional investigation for hematologic complications, specifically thromboembolism, was not pursued. Given his age with no prior history or family history of hypercoagulability, the presence of petechiae, and his oral findings, the lack of an exam consistent with a lower-extremity DVT, the bilateral nature of his lower-extremity pain, the above imaging findings suggestive of an inflammatory process, and the rapid improvement of his symptoms and exam on vitamin C therapy, our differential diagnosis was focused on osteomyelitis versus scurvy. Therefore, our pre-test probability for thromboembolism as the etiology for his GT site necrosis, even during the early part of his hospitalization, was low enough that we did not perform additional hematologic testing.
Discussion
This case of scurvy, to the best of our knowledge, is novel in 3 respects. First, the presentation to care was due to a significant non-oral wound, a late finding in scurvy [10–12].
Second, nearly all prior pediatric case reports describe difficulty or an inability to ambulate, a key sign in the presentation of scurvy, especially in children [3,12,14]. Through a defect in collagen production, there is disordered osteoid matrix formation with poor cartilage resorption, resulting in a defect in bone structure. Subsequently, microfractures near the growth plate may occur, as well as subperiosteal hemorrhage, culminating in bone pain, limp, or refusal to walk, with the lower extremities most affected [1,5,10,12]. Given the higher bone turnover in a growing pediatric patient and with periosteum that is not as tightly bound to the cortex, children have more severe skeletal changes and are more likely to present with bone pain than adults [3,12]. Our patient was non-ambulatory, which likely delayed his time to both presentation and diagnosis. Notably, he had bilateral lower-extremity pain, the severity of which may have been underappreciated due to his speech delay and non-ambulatory status.
Third, the path to diagnosis in this case was unique due to the presence of lower-extremity petechiae without anemia, thrombocytopenia, or coagulopathy, suggesting diagnoses such as von Willebrand disease, Henoch-Schönlein purpura, and rheumatologic disorders. Anemia is common in scurvy [1,5,9] and would narrow this differential significantly; 42% of pediatric scurvy patients present with anemia, likely due to the role of vitamin C in promoting iron absorption [9].
Nutritional deficiencies are often underdiagnosed by pediatricians, creating a need for reform in disease recognition [1–3,8]. We present our lessons learned in the management of scurvy. Based on our experience with this case, we urge clinicians to consider scurvy in patients with lower-extremity pain without fever, with or without accompanying gait alterations. In our opinion, this was a key factor in the diagnostic delay of our patient. They should obtain a full diet history, and if a pattern of selective eating is identified, a vitamin C level should be assessed. Although not specific, the near universality of lower-extremity pain in pediatric scurvy [3,12,14] should prompt providers to consider this diagnosis as part of a broad initial differential.
In the setting of clinical features consistent with scurvy, we recommend forgoing imaging if infection is deemed unlikely. Prior case reports describe imaging findings as inconsistent and note that key scurvy radiographic features often go unrecognized [2,4]. Many of the classic radiologic features can be nonspecific, potentially leading to unnecessary workup or delay in treatment [10].
The classic radiology findings of scurvy are well described in the literature (Figures 2, 3) [1–5,11,24]. In Perkins et al, none of the 9 patients that had plain radiographs of the lower extremities had radiology reports initially suggestive of scurvy [1], consistent with our patient. There may be MRI overlap with osteomyelitis, leading to diagnostic uncertainty and additional workup [5]. However, in patients with high clinical suspicion, MRI may offer a predictable appearance of scurvy, with symmetric areas of T2 hyperintensity and enhancement predominately seen in the long-bone metaphyses [4,24].
Osteomyelitis is often in the differential for scurvy [25]. In the absence of fever with a convincing diet history, we argue that clinicians should have confidence to withhold antibiotics and initiate vitamin C therapy early. Elevated inflammatory markers should not be a barrier to discontinuing antibiotics, as scurvy can cause such laboratory findings [1,5,10,26]. Our patient’s CRP decreased on hospital day 5 (Table 3). The presentation of tenderness, edema, warmth, and elevated inflammatory markers, however, can obfuscate the clinical picture when assessing for an infectious process such as osteomyelitis [27] versus a nutritional deficiency.
While awaiting laboratory confirmation of hypovitaminosis C, clinicians should not hesitate to initiate scurvy-directed therapy in the setting of a convincing clinical and dietary history. These history elements alone are often sufficient for diagnosis. Starting treatment early is diagnostically informative [2,4,10,11], as constitutional and oral symptoms of scurvy can improve within days [11,12]. A diet history and early treatment may be a faster and more powerful tool than invasive testing [5].
Pediatric acute scurvy treatment is 100 mg of oral vitamin C 3 times per day for at least 1 week, then maintenance dosing of 100 mg daily until symptom resolution, generally for at least 1 month [10,11,28]. Bone and bleeding manifestations can take weeks to resolve [11,12]. Concomitant iron, zinc, vitamins B1, B6, B9, B12, and D deficiencies have been well documented in patients with scurvy and warrant investigation and supplementation [1,9–11,28].
At our institution, the vitamin C assay is a send-out lab test, which, in our patient, delayed stopping antibiotic treatment for potential osteomyelitis. Scurvy is not uncommon in tertiary and quaternary pediatric health systems and the incidence is likely rising [2]. Therefore, we advocate for in-house assays for serum vitamin C levels. Early recognition can mitigate the need for additional workup, which is often expensive and invasive [1,2,4,5,10].
Conclusions
Children with nutritional deficiencies appear to be receiving regular pediatric care, yet many are going undiagnosed, suggesting that reform is needed in how general pediatricians assess for nutritional disorders. We advocate for pediatricians to take a thorough diet history at every well-child visit and more frequently for children with any risk factor. Our patient was instructed to discontinue his gastrostomy tube feeds at home, but there was no follow-up ensured for his nutrition. We encourage pediatricians to include nutritional deficiencies in their differential diagnosis for serious illnesses, multisystem presentations, or diagnostic dilemmas. We believe our patient’s diagnosis was delayed by his non-ambulatory status, as most children present with gait abnormalities. Clinicians should consider scurvy in pediatric patients with lower-extremity pain without fever, even if gait changes are not present. Vitamin C therapy should be started early, even if results of laboratory studies are not yet available. Given the increasing incidence, we advocate for children’s hospitals to use on-site serum vitamin C assays, which would have avoided additional testing and therapies in our patient. A clinical presentation and diet history are often sufficient for diagnosis, so extensive diagnostic workup for other potential etiologies may be unnecessary. Additionally, in this setting, antibiotics can be avoided in the absence of fever when an infectious process is a competing diagnosis. In considering the above lessons learned, pediatricians can optimize their management of this increasingly common condition.
Figures
References:
1.. Perkins A, Sontheimer C, Otjen JP, Shenoi S, Scurvy masquerading as juvenile idiopathic arthritis or vasculitis with elevated inflammatory markers: A case series: J Pediatr, 2020; 218; 234-37.e2
2.. Golriz F, Donnelly LF, Devaraj S, Krishnamurthy R, Modern American scurvy – experience with vitamin C deficiency at a large children’s hospital: Pediatr Radiol, 2017; 47(2); 214-20
3.. Duggan CP, Westra SJ, Rosenberg AE, Case 23–2007 – a 9-year-old boy with bone pain, rash, and gingival hypertrophy: N Engl J Med, 2007; 357(4); 392-400
4.. Gulko E, Collins LK, Murphy RC, MRI findings in pediatric patients with scurvy: Skeletal Radiol, 2015; 44(2); 291-97
5.. Harknett KMW, Hussain SK, Rogers MK, Patel NC, Scurvy mimicking osteomyelitis: Case report and review of the literature: Clin Pediatr (Phila), 2013; 53(10); 995-99
6.. Ma NS, Thompson C, Weston S, Brief report: Scurvy as a manifestation of food selectivity in children with autism: J Autism Dev Disord, 2016; 46(4); 1464-70
7.. Seya M, Handa A, Hasegawa D, Scurvy: From a selective diet in children with developmental delay: J Pediatr, 2016; 177; 331
8.. Liuzzo Scorpo M, Corsello G, Maggio MC, Scurvy as an alarm bell of autistic spectrum disorder in the first world: A case report of a 3-year-old girl: Am J Case Rep, 2021; 22; e930583
9.. Hahn T, Adams W, Williams K, Is vitamin C enough? A case report of scurvy in a five-year-old girl and review of the literature: BMC Pediatrics, 2019; 19(1); 74
10.. Nastro A, Rosenwasser N, Daniels SP, Scurvy due to selective diet in a seemingly healthy 4-year-old boy: Pediatrics, 2019; 144(3); e20182824
11.. Agarwal A, Shaharyar A, Kumar A, Scurvy in pediatric age group – a disease often forgotten?: J Clin Orthop Trauma, 2015; 6(2); 101-7
12.. Weinstein M, Babyn P, Zlotkin S, An orange a day keeps the doctor away: Scurvy in the year 2000: Pediatrics, 2001; 108(3); E55
13.. National Institutes of Health: Office of Dietary Supplements; 2021 [updated March 26, 2021November 20, 2021]; Available from: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
14.. Kitcharoensakkul M, Schulz CG, Kassel R, Scurvy revealed by difficulty walking: Three cases in young children: J Clin Rheumatol, 2014; 20(4); 224-28
15.. Joshi R, Gustas-French CN, Fanburg-Smith JC, Scurvy: A rare case in an adult: Skeletal Radiology, 2019; 48(6); 977-84
16.. Guellich A, Tella E, Mahé E, [Scurvy presenting with low-extremity necrotic and purpuric ulcers: Two cases]: Rev Med Interne, 2021; 42(3); 214-17
17.. Bechara N, Flood VM, Gunton JE, A systematic review on the role of vitamin C in tissue healing: Antioxidants (Basel), 2022; 11(8); 1605
18.. Collins N, Nutrition 411: Revisiting vitamin C and wound healing: Ostomy Wound Manage, 2013; 59(9); 12
19.. Pullar JM, Carr AC, Vissers MCM, The roles of vitamin C in skin health: Nutrients, 2017; 9(8); 866
20.. Mahajerin A, Branchford BR, Amankwah EK, Hospital-associated venous thromboembolism in pediatrics: A systematic review and meta-analysis of risk factors and risk-assessment models: Haematologica, 2015; 100(8); 1045-50
21.. Faustino EVS, Raffini LJ, Prevention of hospital-acquired venous thromboembolism in children: A review of published guidelines: Frontiers in Pediatrics, 2017; 5; 9
22.. Bayram Y, Karaca E, Coban Akdemir Z, Molecular etiology of arthrogryposis in multiple families of mostly Turkish origin: J Clin Invest, 2016; 126(2); 762-78
23.. Pehlivan D, Bayram Y, Gunes N, The genomics of arthrogryposis, a complex trait: Candidate genes and further evidence for oligogenic inheritance: Am J Hum Genet, 2019; 105(1); 132-50
24.. Gongidi P, Johnson C, Dinan D, Scurvy in an autistic child: MRI findings: Pediatr Radiol, 2013; 43(10); 1396-99
25.. Kinlin LM, Blanchard AC, Silver S, Morris SK, Scurvy as a mimicker of osteomyelitis in a child with autism spectrum disorder: Int J Infect Dis, 2018; 69; 99-102
26.. Wannamethee SG, Lowe GD, Rumley A, Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis: Am J Clin Nutr, 2006; 83(3); 567-74
27.. Unkila-Kallio L, Kallio MJ, Eskola J, Peltola H, Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children: Pediatrics, 1994; 93(1); 59-62
28.. Fortenberry M, Rucker H, Gaines K, Pediatric scurvy: How an old disease is becoming a new problem: J Pediatr Pharmacol Ther, 2020; 25(8); 735-41
Figures
Tables
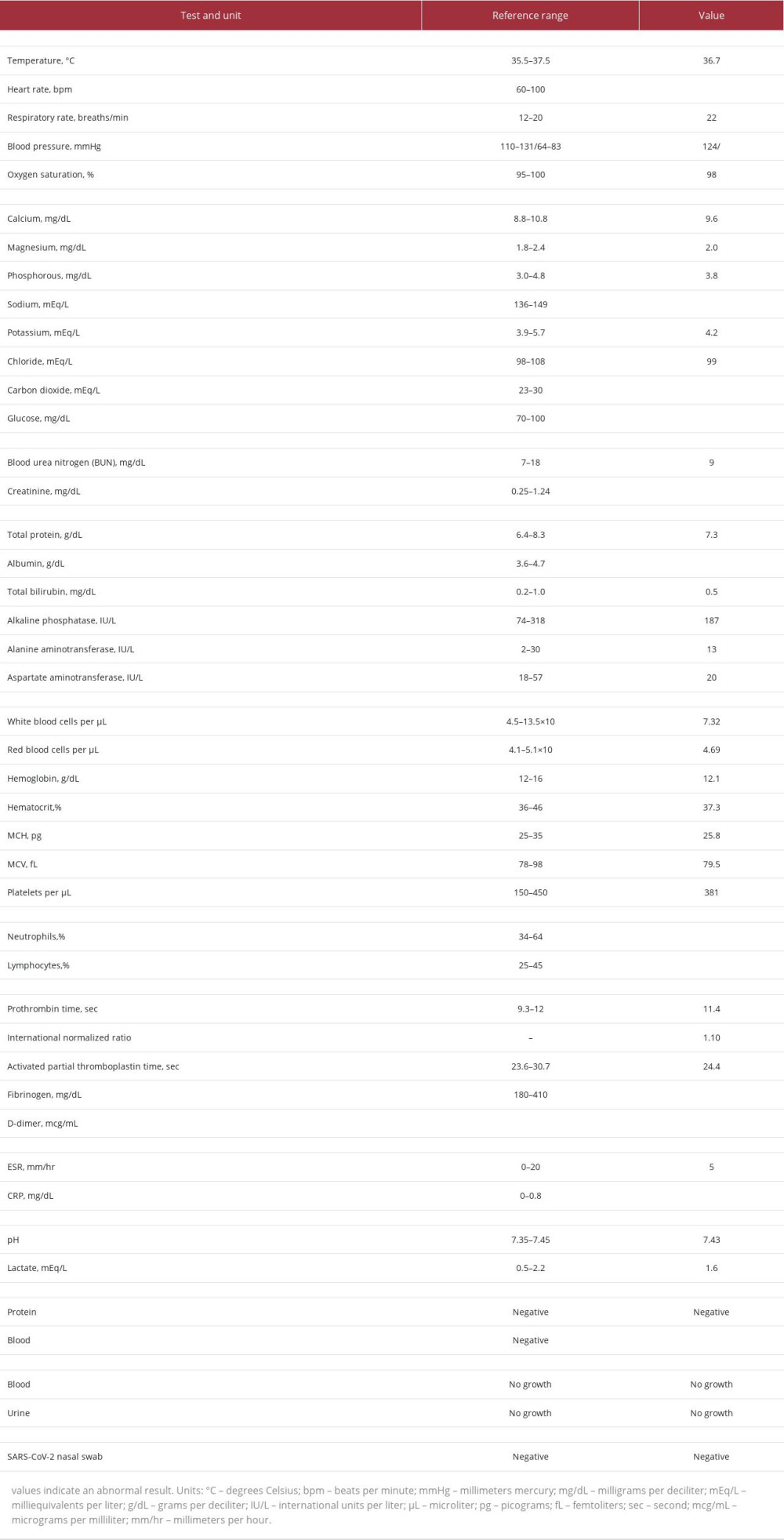 Table 1.. Vital signs and laboratory tests obtained at initial presentation.
Table 1.. Vital signs and laboratory tests obtained at initial presentation.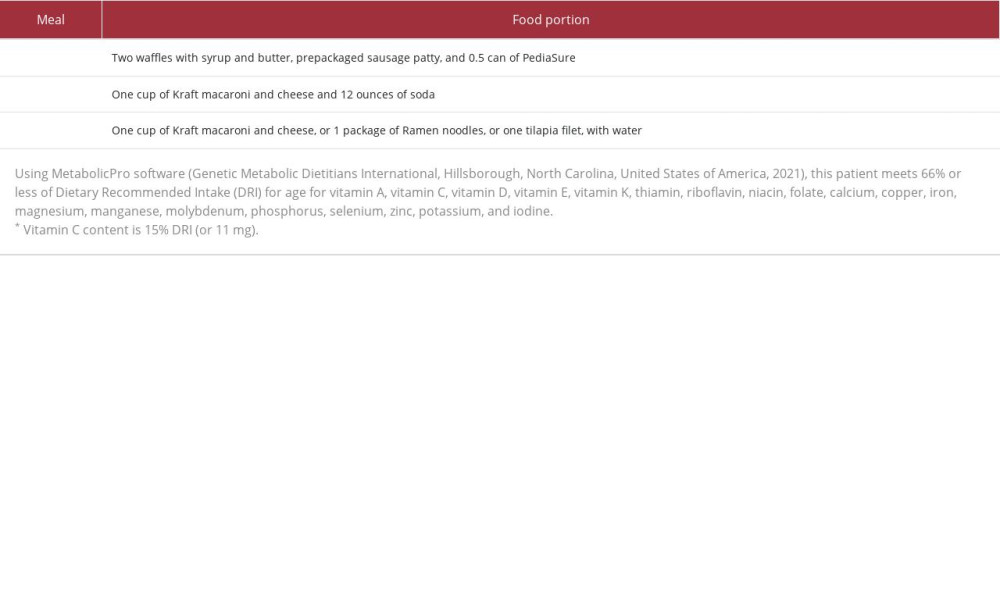 Table 2.. Diet history.
Table 2.. Diet history.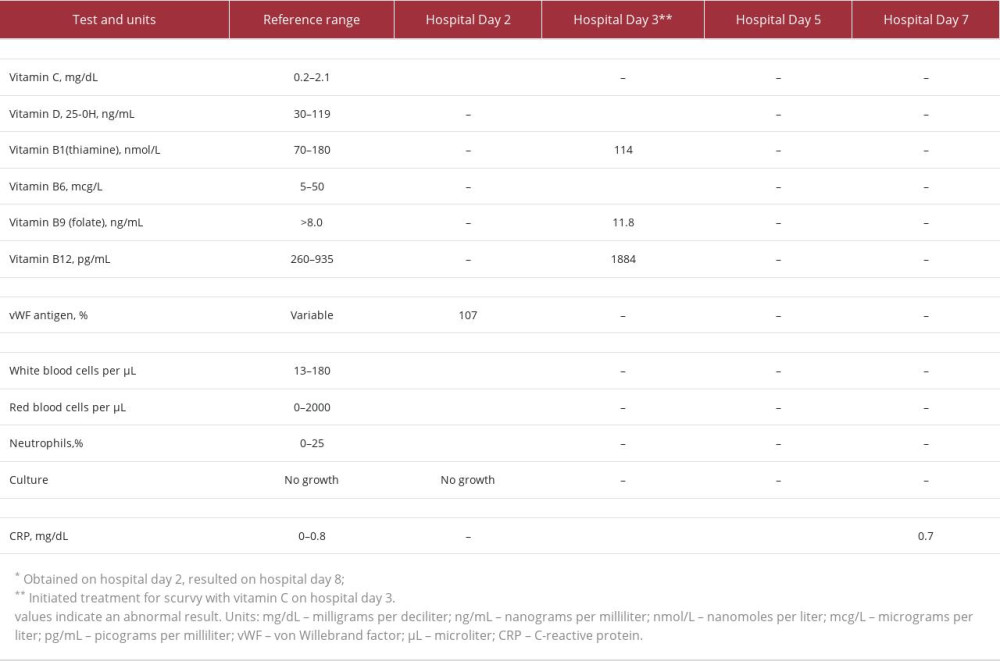 Table 3.. Laboratory testing results during hospital admission.
Table 3.. Laboratory testing results during hospital admission. Table 1.. Vital signs and laboratory tests obtained at initial presentation.
Table 1.. Vital signs and laboratory tests obtained at initial presentation. Table 2.. Diet history.
Table 2.. Diet history. Table 3.. Laboratory testing results during hospital admission.
Table 3.. Laboratory testing results during hospital admission. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








