10 August 2023: Articles 
Successful Hemodialysis Treatment of Severe Hypercalcemia Following COVID-19 in Multiple Myeloma: A Case Report
Unusual clinical course
Christopher H. Goss1AEF*, Shady Ezaldin1AEF, Parisa Aijaz1AEF, Amna Anees1AEFDOI: 10.12659/AJCR.940835
Am J Case Rep 2023; 24:e940835
Abstract
BACKGROUND: Hypercalcemia, a serum calcium exceeding 10.5 mg/dL, is a multi-factorial metabolic disorder that results from an imbalance in calcium homeostasis.
CASE REPORT: We report a case of a 67-year-old male with recently diagnosed multiple myeloma who presented to our emergency department 3 weeks after COVID-19 infection with altered mental status and a fall. On admission he was found to have severe hypercalcemia with a level over 18.0 mg/dL. Despite IV fluids, calcitonin, steroids, and zoledronic acid, he had persistent, critically elevated calcium levels. The decision to initiate hemodialysis was made, which successfully treated his hypercalcemia.
CONCLUSIONS: This report presents a case of malignant hypercalcemia in an individual with COVID-19 and multiple myeloma and highlights the importance of considering dialysis as a viable treatment for hypercalcemia when other modalities have failed.
Keywords: Calcium, COVID-19, Hypercalcemia, Multiple Myeloma, Nephrology, Renal Dialysis, Male, Humans, Aged, COVID-19, Bone Density Conservation Agents
Background
Hypercalcemia is a complex metabolic disorder that results from an imbalance in the regulation of calcium homeostasis [1]. Excess parathyroid hormone (PTH) leads to hypercalcemia [1]. Parathyroid adenomas, familial hypocalciuric hypercalcemia, and multiple endocrine neoplasia are conditions that can lead to hypercalcemia through elevations in PTH [1,2]. In addition to syndromic causes, malignancies are known to cause hypercalcemia [1]. PTH-related peptides can be associated with cancers including renal cell carcinomas, leukemias, and lymphomas [1]. Aberrantly elevated vitamin D levels can lead to hypercalcemia and can be caused by excessive calcium consumption and granulomatous diseases, including sarcoidosis [1]. Endocrine disorders, including hyperthyroidism, hypoadrenalism, and pheochromocytomas, are known to cause hypercalcemia [1]. Less common causes of hypercalcemia can involve medications, including lithium and thiazides, milk-alkali syndrome, fat necrosis, and prolonged immobilization [1].
Routine testing can often detect hypercalcemia [1,2]. Workup for hypercalcemia can involve obtaining serum PTH and calcitonin levels, electrolytes, ionized calcium, vitamin D, alkaline-phosphatase, blood urea nitrogen, creatinine, and urine calcium levels [1,2]. Sensitive and specific assays can be used to measure parathyroid hormone [1,2]. In addition to serum tests, imaging studies including mammogram and computed tomography can be done to rule out less common causes, including sarcoidosis, lung cancer, breast cancer, and renal cancer [1,2]. The parathyroid glands can be observed with ultrasound and magnetic resonance imaging to determine if tumors are present [1,2].
Treatment for hypercalcemia is focused on increasing calcium excretion, reducing gastrointestinal absorption, and decreasing bone resorption [3–5]. Immediate treatment for hypercalcemia involves restoring intravascular volumes by saline or other crystalloid infusion [3,4]. In addition to intravenous (i.v.) fluids, bisphosphonates, zoledronic acid, calcitonin, corticosteroids, denosumab, and diuretics are often used to manage hypercalcemia [3–5]. For patients with parathyroid adenomas/hyperplasia, surgical exploration and excision are definitive treatments. In refractory hypercalcemia, or in patients who cannot tolerate excessive IVF infusion secondary to renal or heart failure, hemodialysis can be used to rapidly reduce serum calcium levels [1].
It is documented that SARS-CoV-2 infection can be associated with hypercalcemia in malignancies, especially hematologic malignancies in the context of prolonged immobility [6]. Kannan et al presented a case of severe hypercalcemia in an immobilized patient recently diagnosed with COVID-19 [7]. The virus that causes COVID-19 has broad tropism and can affect different areas of the body, including the bones and kidneys [8]. COVID-19 can also affect the respiratory system and is known to cause severe respiratory failure and death [9]. One study suggested that the hypothesized mechanism for hyper-calcemia involves the interaction of coronaviruses with the immune system, leading to the generation of cytokines that can activate osteoclasts, leading to bone destruction and subsequent elevations in serum calcium [9]. Another study suggested that SARS-CoV-2 infection may activate various inflammatory mechanisms in the body, leading to hypercalcemia through increased bone resorption and decreased excretion of calcium by the kidneys [10]. Ultimately, while additional research is needed to further assess the relationship between COVID-19 and hypercalcemia in malignancy, the connection between coronavirus activating the immune system and causing aberrant changes in calcium physiology is apparent. This report is of a 67-year-old man with recently diagnosed multiple myeloma who presented to our hospital with altered mental status, reduced mobility, and a fall 3 weeks after COVID-19, who was diagnosed with hypercalcemia that was refractory to typical treatments, and was ultimately treated with hemodialysis.
Case Report
A 67-year-old man recently diagnosed with multiple myeloma presented to the Emergency Department after sustaining a mechanical fall on his right side. He was alert but oriented only to person and place, a deviation from his baseline. As per the family, he had progressive weakness, worsening confusion, reduced mobility, loss of appetite for the previous week, and a subsequent fall leading to his presentation. He had a similar presentation 3 months prior and was diagnosed with multiple myeloma. This patient’s multiple myeloma was diagnosed at an outlying facility, and at the time of presentation there was no access to relevant histopathologic images related to his multiple myeloma or hypercalcemia. He was receiving chemotherapy with bortezomib and daratumumab up until 3 weeks prior, when he was diagnosed with COVID-19. The SARS-CoV-2 infection was diagnosed by reverse transcription-polymerase chain reaction (RT-PCR), and the sample was collected by nasopharyngeal swab. He did not require admission for COVID-19, did not receive any inpatient medical treatment, and was treated conservatively with a few days of bedrest. Leading to his presentation to our hospital, the patient had experienced reduced mobility related to his chronic illnesses, multiple myeloma, and recent COVID-19 disease.
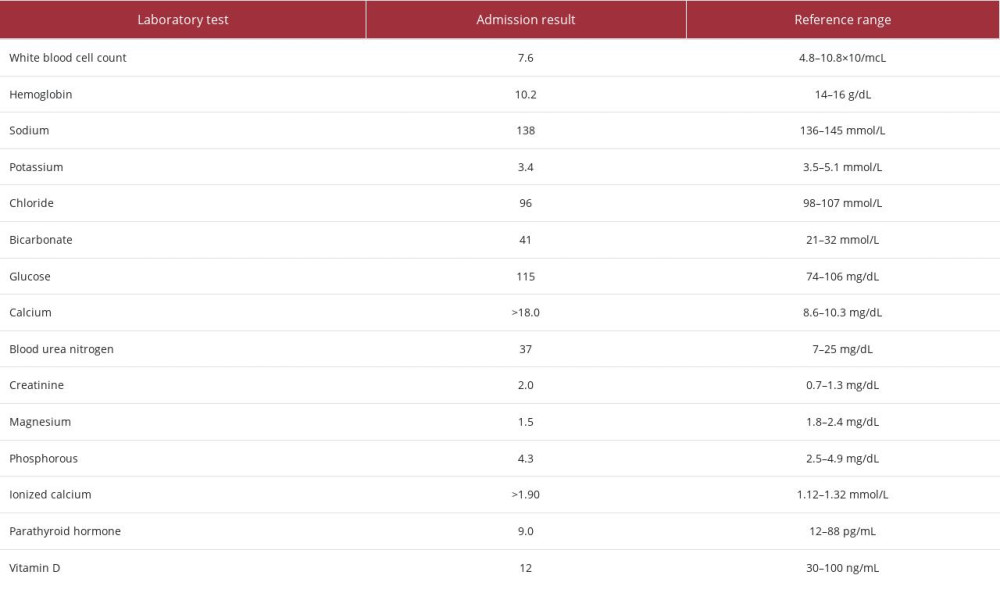
On current admission, the patient was noted to have critical hypercalcemia, with a calcium level greater than 18.0 mg/dL (reference range: 8.6–10.3 mg/dL). The workup for his hypercalcemia included measurement of his parathyroid hormone, vitamin D, and other electrolytes, as noted in Table 1. His parathyroid hormone and vitamin D levels were low, and his electrolyte levels were mostly within normal limits. An acute kidney injury was noted on his metabolic panel. His ionized calcium level was elevated, and albumin level was normal. Urinalysis results were positive for proteinuria, pyuria, and calcium oxalate crystals. Imaging studies, including chest radiography and computerized tomography scans of the head, cervical spine, and chest were negative for any pathologies relevant to the hypercalcemia. Electrocardiography revealed tachycardia and atrial fibrillation. He received 1 dose of zoledronic acid 3.5 mg i.v., calcitonin 400 units subcutaneously, followed by an additional 800 units every 12 h for 2 days, and dexamethasone 20 mg i.v. daily for 2 days. He also received two 500-mL Lactated Ringer’s boluses i.v., and normal saline infusion i.v. at 100 cc/h. Despite these therapies, the patient showed persistently elevated serum calcium levels and T-wave abnormalities on electrocardiogram (EKG). The decision was made to initiate dialysis, and after the initial session of hemodialysis, the patient’s serum calcium level decreased to 14.0 mg/dL, the EKG abnormalities reverted to normal, and his mentation improved significantly. He continued to receive intermittent hemodialysis throughout his hospitalization. The hypercalcemia eventually resolved, and his mentation returned to baseline. His calcium level at discharge was 9.7 mg/dL. Due to persistently elevated creatinine levels and worsening kidney function, permanent access was achieved, and to date, the patient continues to receive hemodialysis as an outpatient.
Discussion
Physical debility and immobility have been associated with the development of hypercalcemia in patients with multiple myeloma who develop COVID-19 infection, although it is an atypical cause of hypercalcemia [7,11]. In cases of refractory hypercalcemia, hemodialysis can be considered as a treatment option [11]. Hypercalcemia can be caused by several conditions, including hyperparathyroidism, malignancies, and various endocrinopathies [11]. Hypercalcemia is often observed in the later stages of solid tumors and hematologic malignancies [11]. Hypercalcemia in multiple myeloma is a complex process involving increased bone turnover and calcium reabsorption in renal tubules, with the severity of the condition correlating with the extent of tumor burden [11]. In our patient, he was recently diagnosed with multiple myeloma, and his chemotherapy was on hold. Additionally, he had a recent SARS-CoV-2 infection and reduced physical mobility secondary to his multiple illnesses. The combination of these factors likely contributed to hypercalcemia. Malignancy-associated hypercalcemia is often caused by the secretion of parathyroid hormone-related protein; however, this is not necessarily a consistent finding in patients with multiple myeloma [12].
Hypercalcemia has recently been linked to COVID-19, but the exact mechanism of this association remains poorly understood [13]. Although most reported cases have occurred in settings of immobilization or rhabdomyolysis due to critical illness, hypercalcemia after COVID-19 has not been commonly observed in cases of malignancy [14,15]. Symptomatic hypercalcemia has been recognized with both multiple myeloma and immobility in patients with COVID-19 [6,7].
The primary treatment for hypercalcemia is i.v. crystalloid infusion, which helps increase urinary calcium excretion, reduce serum calcium levels through dilution, and treat associated hypovolemia [3]. Along with fluids, medications, such as diuretics, bisphosphonates, calcitonin, denosumab, and steroids, can be used to treat hypercalcemia. Loop diuretics increase urinary excretion of calcium to lower blood calcium levels. Bisphosphonates inhibit osteoclast-mediated bone resorption, and calcitonin, a thyroid hormone produced by C cells, can be administered through injection or nasal spray to inhibit bone resorption, and increase urinary calcium excretion [4,16].
Denosumab, a monoclonal antibody infusion, reduces bone resorption and inhibits osteoclast function, which helps prevent hypercalcemia from worsening [5]. Glucocorticoids can also be used to treat hypercalcemia by reducing tumor burden, for example in patients with myeloma [17]. However, in our case, the patient’s hypercalcemia remained resistant to i.v. fluids, steroids, zoledronic acid, and calcitonin. The patient showed EKG changes and altered mentation, requiring urgent and aggressive intervention, including dialysis.
Kannan et al presented a similar case of a patient with prolonged immobility and recent SARS-CoV-2 infection who presented to their hospital with symptomatic severe hypercalcemia. In that case, the patient had prolonged immobility secondary to a complicated hospital course, during which she required extracorporeal membrane oxygenation and intermittent hemodialysis for acute hypoxemic respiratory failure and septic shock related to the SARS-CoV-2 infection [7]. In contrast, our patient presented with symptomatic hypercalcemia in the context of multiple myeloma, recent SARS-CoV-2 infection, and reduced mobility secondary to chronic illness and debility.
The potential benefits of dialysis in treating severe hypercalcemia are often underestimated, even though it can be effective and prompt. Currently, there are no established guidelines for initiating dialysis in hypercalcemia, and the available evidence regarding its efficacy is still developing [17]. To remove excess calcium from the blood in hypercalcemia, the dialysate solution can be modified to contain lower levels of calcium, or none. Citrate can also be used to facilitate calcium removal through the dialyzer [18,19]. However, modifications to conventional dialysis solutions can be necessary for patients with hypercalcemia to avoid exacerbating other metabolic abnormalities, such as hypophosphatemia [20].
Hypercalcemia is relatively common and can be seen in up to 10% to 30% of solid and hematologic malignancies, with the highest rates reported in multiple myeloma [21,22]. In many cases, traditional treatments involving medications and fluids may not be effective, making dialysis a crucial option for managing this condition [11,23,24]. Our patient responded well to dialysis treatment, which proved effective even after other first-line treatments failed. The successful outcome in this case highlights the importance of considering dialysis as a treatment option for patients with critical hypercalcemia. Physicians and other healthcare providers should be aware of the potential benefits of dialysis in these cases and mindful of considering dialysis as a treatment option earlier in the disease course.
Conclusions
This report has highlighted the importance of early diagnosis and management of hypercalcemia and raised awareness of its association with hematologic malignancies, including multiple myeloma, and immobility that can be associated with COVID-19. This case also highlights the critical role of timely dialysis in the management of intractable hypercalcemia that does not respond to first-line therapies.
References:
1.. Sadiq NM, Naganathan S, Badireddy M, Hypercalcemia. [Updated 2022 Sep 5].: StatPearls [Internet]. Jan, 2023, Treasure Island fl, StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK430714/
2.. Reid L, Muthukrishnan B, Patel D, Presentation, diagnostic assessment and surgical outcomes in primary hyperparathyroidism: A single centre’s experience.: Endocr Connect, 2018; 7(10); 1105-15
3.. Makras P, Papapoulos SE, Medical treatment of hypercalcaemia: Hormones (Athens), 2009; 8(2); 83-95
4.. Singer FR, Ritch PS, Lad TE, Treatment of hypercalcemia of malignancy with intravenous etidronate. A controlled, multicenter study. The Hypercalcemia Study Group [published correction appears in Arch Intern Med. 1991;151[10]: 2008].: Arch Intern Med, 1991; 151(3); 471-76
5.. Dietzek A, Connelly K, Cotugno M, Denosumab in hypercalcemia of malignancy: A case series: J Oncol Pharm Pract, 2015; 21(2); 143-47
6.. Vakiti A, Anastasopoulou C, Mewawalla P, Malignancy-related hypercalcemia. [Updated 2023 Apr 16].: StatPearls [Internet]. Jan, 2023, Treasure Island fl, StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK482423/
7.. Kannan L, Raj R, Rhoad W, Immobilization-induced hypercalcemia in COVID-19 with a prolonged Intensive Care Unit stay.: Cureus, 2022; 14(4); e24081
8.. Rosner MH, Dalkin AC, Onco-nephrology: The pathophysiology and treatment of malignancy-associated hypercalcemia: Clin J Am Soc Nephrol, 2012; 7(10); 1722-29
9.. Arif A, Shakir N, Abu-Limon A, Abstract #1014122: COVID-19 associated hypercalcemia, a rare yet interesting case: Endocr Pract, 2021; 27(6); S105-S6
10.. Steenblock C, Schwarz PEH, Ludwig B, COVID-19 and metabolic disease: Mechanisms and clinical management: Lancet Diabetes Endocrinol, 2021; 9(11); 786-98
11.. Basok AB, Rogachev B, Haviv YS, Vorobiov M, Treatment of extreme hypercalcaemia: The role of haemodialysis.: BMJ Case Rep., 2018; 2018 bcr2017223772
12.. Oyajobi BO, Multiple myeloma/hypercalcemia.: Arthritis Res Ther, 2007; 9(Suppl. 1); S4
13.. Bentata Y, Benabdelhak M, Haddiya I, Severe hypercalcemia requiring acute hemodialysis: A retrospective cohort study with increased incidence during the COVID-19 pandemic.: Am J Emerg Med, 2022; 51; 374-77
14.. Li APZ, Thomas S, Gokmen R, Kariyawasam D, Rhabdomyolysis and severe biphasic disturbance of calcium homeostasis secondary to COVID-19 infection: BMJ Case Rep, 2021; 14(5); e239611
15.. Mesland JB, Collienne C, Laterre PF, Hantson P, Immobilization-related hypercalcemia in a COVID-19 patient with prolonged Intensive Care Unit stay: Am J Phys Med Rehabil, 2022; 101(1); 61-63
16.. Chevallier B, Peyron R, Basuyau JP, [Human calcitonin in neoplastic hypercalcemia. Results of a prospective randomized trial.]: Presse Med., 1988; 17(45); 2375-77
17.. Chouhani B, Allata Y, Chouhani W, Hypercalcemia: Is dialysis still an option?: Open J Endocr Metab Dis, 2022; 12; 103-11
18.. Camus C, Charasse C, Jouannic-Montier I, Calcium free hemodialysis: Experience in the treatment of 33 patients with severe hypercalcemia: Intensive Care Med, 1996; 22(2); 116-21
19.. Koo WS, Jeon DS, Ahn SJ, Calcium-free hemodialysis for the management of hypercalcemia: Nephron, 1996; 72(3); 424-28
20.. Leehey DJ, Ing TS, Correction of hypercalcemia and hypophosphatemia by hemodialysis using a conventional, calcium-containing dialysis solution enriched with phosphorus: Am J Kidney Dis, 1997; 29(2); 288-90
21.. Gastanaga VM, Schwartzberg LS, Jain RK, Prevalence of hypercalcemia among cancer patients in the United States: Cancer Med, 2016; 5(8); 2091-100
22.. Stewart AF, Clinical practice. Hypercalcemia associated with cancer.: N Engl J Med, 2005; 352(4); 373-79
23.. Loh HH, Mohd Noor N, The use of hemodialysis in refractory hypercalcemia secondary to parathyroid carcinoma: Case Rep Crit Care, 2014; 2014; 140906
24.. Kaiser W, Biesenbach G, Kramar R, Zazgornik J, Calcium free hemodialysis: An effective therapy in hypercalcemic crisis – report of 4 cases: Intensive Care Med, 1989; 15(7); 471-74
25.. Buege MJ, Do B, Lee HC, Corrected calcium versus ionized calcium measurements for identifying hypercalcemia in patients with multiple myeloma: Cancer Treat Res Commun, 2019; 21; 100159
26.. Bentata Y, Benabdelhak M, Haddiya I, Severe hypercalcemia requiring acute hemodialysis: A retrospective cohort study with increased incidence during the COVID-19 pandemic: Am J Emerg Med, 2022; 51; 374-77
In Press
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942921
22 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943346
24 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943560
26 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943893
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250