30 August 2023: Articles 
Bevacizumab-Associated Thrombotic Microangiopathy Treated with Eculizumab: A Case Report
Challenging differential diagnosis, Unusual setting of medical care, Rare disease, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Wallace Stwart Carvalho PadilhaDOI: 10.12659/AJCR.940906
Am J Case Rep 2023; 24:e940906
Abstract
BACKGROUND: Bevacizumab is an approved targeted therapy for metastatic cancer treatment. It can have adverse effects on multiple organs. Despite its low incidence, thrombotic microangiopathy (TMA) is the most severe complication. TMA has been associated with complement dysregulation, and treatment with eculizumab can be effective, despite the paucity of literature on eculizumab therapy for bevacizumab-associated TMA. To date, 10 cases have been reported, with less than half of them including a kidney biopsy. We present a new case of bevacizumab-associated TMA successfully treated with eculizumab, along with kidney biopsy records and an overview of mechanisms underlying TMA development in bevacizumab-treated patients.
CASE REPORT: A female patient diagnosed with metastatic breast cancer who was treated with bevacizumab in conjunction with chemotherapy was admitted to the hospital for acute kidney injury requiring hemodialysis, microangiopathic hemolytic anemia, and thrombocytopenia. TMA was diagnosed and was later confirmed by a kidney biopsy. Primary causes for TMA, such as ADAMTS13 deficiency and shiga toxin associated hemolytic-uremic syndrome, were ruled out, and the patient’s condition was ultimately found to be triggered by exposure to bevacizumab. After discontinuing bevacizumab and receiving 4 weekly doses of eculizumab, kidney function and hematological parameters improved.
CONCLUSIONS: Bevacizumab-associated TMA can be reversed or attenuated in some patients with the use of eculizumab (inhibiting complement system overactivation), possibly reducing time to recovery, with fewer long-term sequelae. This additional case encourages future clinical trials to evaluate the safety and efficacy of eculizumab in cases of TMA associated with bevacizumab.
Keywords: Atypical Hemolytic Uremic Syndrome, bevacizumab, eculizumab, Acute Kidney Injury, Thrombotic Microangiopathies, Humans, Female, Antibodies, Monoclonal, Humanized, Purpura, Thrombotic Thrombocytopenic
Background
Bevacizumab is a monoclonal antibody drug that inhibits the activity of vascular endothelial growth factor (VEGF), a protein that promotes angiogenesis, the most important step in the development and progression of malignant tumors. In some countries, bevacizumab is approved for the treatment of advanced cancer, including metastatic breast cancer, in conjunction with chemotherapy [1].
Despite its efficacy, bevacizumab is frequently associated with adverse effects [1]. Proteinuria and hypertension are the most common kidney adverse effects, while a minority of patients can develop thrombotic microangiopathy (TMA), a more severe complication.
Bevacizumab-associated TMA, along with other drug-induced TMAs, are currently an indication for drug discontinuation, owing to a poor prognosis, namely the association with acute kidney injury, which often requires dialysis, and progression to chronic kidney disease. Most cases improve after discontinuation of the drug [2], but remaining cases persist and continue to deteriorate despite stopping bevacizumab [3]. Aside from drug discontinuation and supportive care, no other treatment has reached consensus [1]. Considering the role of complement dysregulation in bevacizumab-associated TMA, case series and reports [3,4] suggest that eculizumab is a promising therapy. Literature on eculizumab treatment of bevacizumab-associated TMA is limited, as only 10 cases have been reported [3–9], of which only 4 have a kidney biopsy description.
In this article, we report the eleventh case of a patient with advanced breast cancer who developed bevacizumab-associated TMA, was treated with eculizumab, and achieved a favorable kidney outcome. We briefly discuss the main known mechanisms leading to TMA in bevacizumab-treated patients.
Case Report
A 64-year-old woman with a history of mild hypertension underwent elective upper gastric endoscopy owing to dyspeptic symptoms. A biopsy of a gastric lesion revealed poorly differentiated carcinoma. She was referred to an oncologist, and after a complete workup, the gastric lesion was identified as a metastasis from a hormonal receptor-positive, Her2-negative primary lobular breast cancer. She also had lymph node metastasis, and a final diagnosis of stage IV breast cancer was made.
First-line treatment was fulvestrant, but as the disease progressed, she began second-line treatment with palbociclib and letrozole until August 2021. Owing to a new progression manifesting as a visceral crisis, she began paclitaxel and bevacizumab (10mg/kg every 3 weeks) in September 2021.
Prior to receiving bevacizumab, her estimated glomerular filtration rate at baseline by 2021 CKD-EPI equation was greater than 90 mL/min/1.73 m2 (creatinine 0.60 mg/dL), and there was no proteinuria. In the following months, her blood pressure began to rise, and she developed lower limb edema. Regular laboratory tests revealed that her creatinine level was gradually rising. She was hospitalized 7 months after initiating bevacizumab for refractory hypertension, worsening edema, and deteriorating kidney function (creatinine of 1.32 mg/dL, as shown in Table 1).
Initial assessments revealed evidence of non-immune microangiopathic hemolytic anemia (negative direct Coombs test, elevated lactate dehydrogenase levels, low haptoglobin levels, and the presence of 4% schistocytes on peripheral blood smear) and thrombocytopenia. The complement levels (C3, 111 mg/dL, and C4, 36 mg/dL) and ADAMTS13 levels (99%) were within the normal range (Table 1). ADAMTS13 antibody and other complement assays, such as C5b9 or functional tests, were not available. Both ANA and ANCA were negative. Kidney assessment revealed sub-nephrotic proteinuria (1242 mg/24 h) and acute kidney injury (creatinine level increased from 0.60 to 1.32 mg/dL). Clinically, she had generalized edema (anasarca) and refractory hypertension (despite the use of 5 different medications, including diuretics).
Due to kidney dysfunction and signs of TMA, bevacizumab was promptly discontinued. She was prescribed furosemide for anasarca and higher antihypertensive drug dosages for hypertension. A kidney biopsy was performed, when blood pressure levels were permissible. Figures 1 and 2 describe the confirmatory findings of TMA.
Two days after the biopsy, her kidney function continued to deteriorate, and she started hemodialysis treatment. After the first session of hemodialysis, eculizumab was initiated at a dosage of 900 mg intravenously every week for 4 weeks. She had her eleventh and last dialysis session 1 day before her last dose of eculizumab, followed by progressive improvement of laboratory abnormalities. Two weeks after discharge, her creatinine levels continued to drop and reached a new baseline of 1.36 mg/dL. Proteinuria remained elevated in the sub-nephrotic range, and she continued to rely on 4 medications to control her blood pressure.
Discussion
We report the case of a woman with bevacizumab-treated stage IV breast cancer who developed TMA secondary to bevacizumab exposure and presented with systemic and renal disease: microangiopathic hemolytic anemia, thrombocytopenia, and acute kidney injury requiring hemodialysis. She discontinued bevacizumab, but due to deteriorating kidney function, she was given 4 weekly doses of 900 mg eculizumab. On the fourth week, she no longer required hemodialysis and achieved normal haptoglobin levels with absence of schistocytes on peripheral blood smear.
Eculizumab is a monoclonal antibody that blocks the cleavage of C5 into C5a, a key process in the complement cascade that leads to the formation of the membrane attack complex C5b-9. It is used successfully in primary complement-mediated hemolytic uremic syndromes, and it has been used in cases of chemotherapy-induced TMA [6,8], mostly with gemcitabine and mitomycin-C, although its indication and efficacy in this context remain uncertain. Currently, the standard treatment for drug-induced TMA consists only of drug discontinuation and supportive care [10].
To date, only 10 cases of bevacizumab-associated TMA treated with eculizumab have been reported. Table 2 shows a summary of 11 cases, including our present case report. Of the total, 90% of previous cases in female patients, and their ages ranged from 17 to 74 years (median=65). The most prevalent primary site cancer was ovarian. Based on 6 cases, the average weekly dose of bevacizumab was 4.3 mg/kg/week. The protocol for eculizumab was described in 9 cases, all of which initially targeted 900 mg weekly for 4 weeks; after this period, 7 patients continued the medication on a variable dose and frequency schedule. Only 1 patient was resistant to eculizumab; all others showed varying degrees of improvement in kidney function (none required dialysis). In our case, the bevacizumab weekly dose was 3.3 mg/kg/week, 22% lower than average. The initial regimen for eculizumab was the same as in previous cases, consisting of 900 mg weekly for 4 weeks, with no additional doses required, because complete hematological and partial renal responses had been achieved (with the patient no longer requiring dialysis).
The rationale for treating bevacizumab-associated TMA with eculizumab comes from the fact that bevacizumab disrupts VEGF’s function, resulting in endothelial and complement system disturbances, which are both implicated in TMA physiopathology. In the glomerulus, podocytes are the primary source of VEGF, producing high levels of VEGF constitutively [11]. Standard treatment with discontinuation of bevacizumab will eventually correct VEGF function, reversing endothelial and complement dysfunctions in most cases. However, remaining cases that do not improve may benefit from eculizumab, as these patients may have a predisposition to overactivation of the alternative complement system pathway, which is triggered by bevacizumab, perpetuating TMA’s effects.
One of the mechanisms for endothelial dysfunction includes the loss of endothelial nitric oxide synthase upregulation (due to less VEGF receptor-2 activation), with consequently less production of nitric oxide [12], leading to higher vascular tone and vasoconstriction and less inhibition of platelet adhesion and aggregation, possibly promoting microvascular thrombi formation and tissue ischemia. Another mechanism described is the loss of fenestrations of glomerular endothelial cells, which is normally induced by VEGF [11, 13]. Without this healthy fenestrated phenotype, microvascular injury and thrombotic microangiopathy can occur [11].
The knowledge that local podocyte-derived VEGF regulates local complement activity by inducing complement factor H (CFH) synthesis in the glomerulus led to the understanding that bevacizumab might cause complement system dysfunction in the kidneys [14]. CFH is a soluble glycoprotein expressed in both podocytes and glomerular endothelial cells of the kidney, and it regulates the complement system by inhibiting the spontaneous activation of the alternative pathway [15]. Therefore, bevacizumab reduces glomerular VEGF and decreases CFH local expression, resulting in spontaneous activation of the alternative complement pathway with the formation of membrane attack complex and endothelial and podocyte injury, further perpetuating the process [14,16]. The treatment with eculizumab may halt the overactivation of the complement system and discontinue tissue damage. Theoretically, if administered early in the course of the condition, it could reduce the severity of kidney damage and shorten the time required for kidney function to recover, possibly resulting in less tissue fibrosis and a lower incidence of chronic kidney disease.
It is important to highlight the significant challenge that several biases impose on the analysis of cases like this. Unfortunately, we were unable to investigate possible mutations in the complement pathway that may have contributed to the case (given that carriers of CFH genetic variants may be more sensitive to VEGF antagonism [14]), nor to state with no doubt that bevacizumab was the only cause of TMA, given the wide range of medications to which oncologic patients are exposed, including concurrent chemotherapy, and the fact that cancer itself is also a cause of secondary TMA. In addition, we cannot generalize these findings since only the discontinuation of medication and the course of time could account for the clinical improvement, regardless of the use of eculizumab. Therefore, additional research is needed before definitive conclusions can be drawn.
Conclusions
Although TMA is a rare adverse effect in oncologic patients undergoing treatment with the targeted therapy bevacizumab, its occurrence is associated with high morbidity and mortality and no treatment other than supportive care and discontinuation of bevacizumab. With the growing population of oncologic patients on anti-VEGF therapy, we should search for better treatment to alleviate the burden of potential outcomes, such as the need for chronic dialysis. Here we present one new case that, combined with the previous 10 cases of literature, consists of the initial data to support a future clinical trial to test eculizumab’s safety and efficacy for bevacizumab-associated TMA. This case is also a learning opportunity for clinicians to be aware of this potential adverse reaction (TMA) when treating patients with anti-VEGF drugs.
Figures
References:
1.. Perazella MA, Izzedine H, New drug toxicities in the onco-nephrology world: Kidney Int, 2015; 87(5); 909-17
2.. Usui J, Glezerman IG, Salvatore SP, Clinicopathological spectrum of kidney diseases in cancer patients treated with vascular endothelial growth factor inhibitors: A report of 5 cases and review of literature: Hum Pathol, 2014; 45(9); 1918-27
3.. Hilburg R, Geara AS, Qiu MK, Bevacizumab-associated thrombotic microangiopathy treated with eculizumab: A case series and systematic review of the literature: Clin Nephrol, 2021; 96(1); 51-59
4.. İnözü M, Özlü SG, Özyörük D, Eculizumab for bevacizumab induced thrombotic microangiopathy: A case report: Pediatr Hematol Oncol J, 2022; 7(4); 169-72
5.. Al Ustwani O, Lohr J, Dy G, Eculizumab therapy for gemcitabine induced hemolytic uremic syndrome: Case series and concise review: J Gastrointest Oncol, 2014; 5(1); E30-33
6.. Weitz IC, Deloughery T, Effective treatment of chemotherapy induced atypical Haemolytic Uraemic Syndrome: A case series of 7 treated patients: Br J Haematol, 2018; 183(1); 136-39
7.. Vakiti A, Singh D, Pilla R, Alhaj-Moustafa M, Fitzpatrick KW, Bevacizumab-induced atypical hemolytic uremic syndrome and treatment with eculizumab: J Oncol Pharm Pract, 2019; 25(4); 1011-15
8.. Hausberg M, Felten H, Pfeffer S, Treatment of chemotherapy-induced thrombotic microangiopathy with eculizumab in a patient with metastatic breast cancer: Case Rep Oncol, 2019; 12(1); 1-6
9.. Viscardi G, Zanaletti N, Ferrara MG, Atypical haemolytic-uraemic syndrome in patient with metastatic colorectal cancer treated with fluorouracil and oxaliplatin: A case report and a review of literature: ESMO Open, 2019; 4(5); e000551
10.. Genest DS, Patriquin CJ, Licht C, Renal thrombotic microangiopathy: A review: Am J Kidney Dis, 2023; 81(5); 591-605
11.. Eremina V, Jefferson JA, Kowalewska J, VEGF inhibition and renal thrombotic microangiopathy: N Engl J Med, 2008; 358(11); 1129-36
12.. Kroll J, Waltenberger J, VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR): Biochem Biophys Res Commun, 1998; 252(3); 743-46
13.. Pfister F, Amann K, Daniel C, Characteristic morphological changes in anti-VEGF therapy-induced glomerular microangiopathy: Histopathology, 2018; 73(6); 990-1001
14.. Keir LS, Firth R, Aponik L, VEGF regulates local inhibitory complement proteins in the eye and kidney: J Clin Invest, 2017; 127(1); 199-214
15.. Kopp A, Hebecker M, Svobodová E, Józsi M, Factor h: A complement regulator in health and disease, and a mediator of cellular interactions: Biomolecules, 2012; 2(1); 46-75
16.. Estrada CC, Maldonado A, Mallipattu SK, Therapeutic inhibition of VEGF signaling and associated nephrotoxicities: J Am Soc Nephrol, 2019; 30(2); 187-200
Figures
Tables
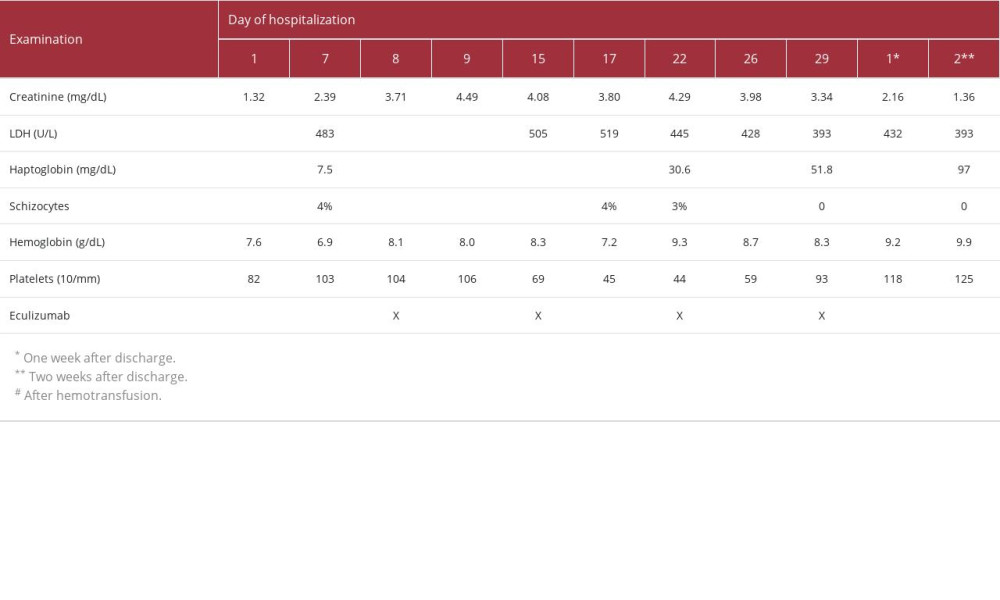 Table 1.. Timeline of laboratory examinations and eculizumab infusion.
Table 1.. Timeline of laboratory examinations and eculizumab infusion.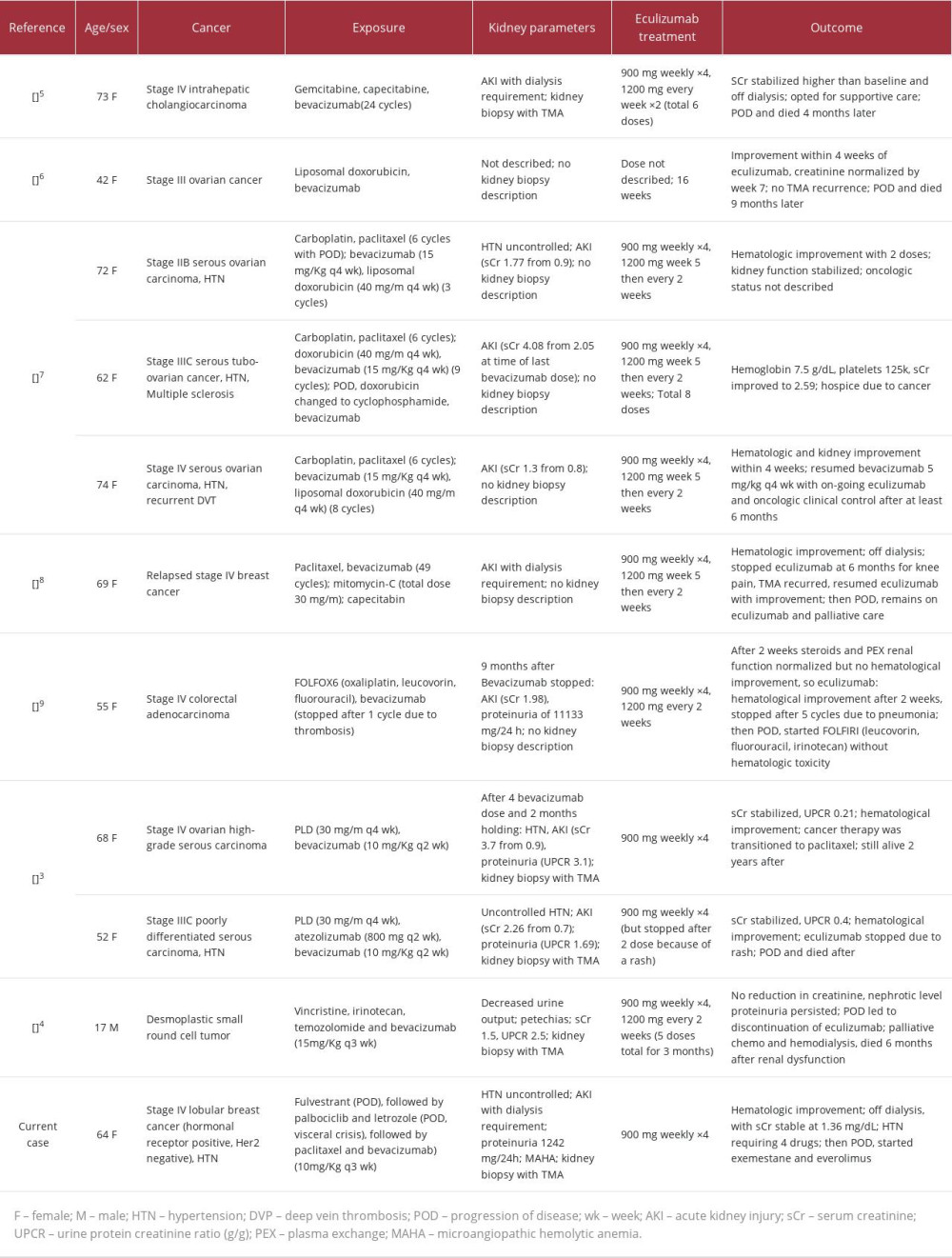 Table 2.. Summary of case reports of bevacizumab-induced TMA treated with eculizumab.
Table 2.. Summary of case reports of bevacizumab-induced TMA treated with eculizumab. Table 1.. Timeline of laboratory examinations and eculizumab infusion.
Table 1.. Timeline of laboratory examinations and eculizumab infusion. Table 2.. Summary of case reports of bevacizumab-induced TMA treated with eculizumab.
Table 2.. Summary of case reports of bevacizumab-induced TMA treated with eculizumab. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








