20 September 2023: Articles 
Eosinophilic Granulomatosis with Polyangiitis Presenting as Steroid-Responsive Sclerosing Cholangitis and Cholecystitis: A Rare Case Report
Unusual clinical course
Yuhei FujisawaDOI: 10.12659/AJCR.940990
Am J Case Rep 2023; 24:e940990
Abstract
BACKGROUND: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare systemic vasculitic condition characterized by bronchial asthma and eosinophilia. While biliary involvement is uncommon in EGPA, we present a unique case of EGPA presenting as steroid-responsive sclerosing cholangitis and cholecystitis. This case highlights the importance of considering EGPA in the differential diagnosis of biliary diseases, especially in patients with a history of bronchial asthma.
CASE REPORT: A 47-year-old man with a history of bronchial asthma presented with fatigue, weight loss, and epigastralgia. Blood tests revealed eosinophilia and elevated inflammatory markers, leading to the diagnosis of EGPA. Further imaging studies, including magnetic resonance cholangiopancreatography and contrast-enhanced computed tomography, confirmed the presence of sclerosing cholangitis and cholecystitis, a rare manifestation of EGPA.
CONCLUSIONS: Prompt treatment with prednisolone and azathioprine resulted in remission of symptoms and resolution of cholangitis and cholecystitis in this case. Our findings emphasize the importance of early recognition and appropriate management of EGPA-associated biliary involvement. Increased awareness of this rare manifestation may facilitate timely diagnosis and improve patient outcomes.
Keywords: Cholangitis, Cholecystitis, Churg-Strauss syndrome, Prednisolone, Male, Humans, Middle Aged, Cholangitis, Sclerosing, granulomatosis with polyangiitis, Asthma, Rare Diseases
Background
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a rare vasculitis syndrome characterized by bronchial asthma and eosinophilia. It falls within the spectrum of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides, sharing similarities with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). EGPA is characterized by inflammation and tissue damage in small- to medium-sized blood vessels, leading to multi-organ involvement, including the lungs, heart, kidneys, and gastrointestinal tract.
The hallmark clinical triad of EGPA consists of asthma, eosinophilia, and systemic vasculitis, accompanied by a wide range of extra-pulmonary manifestations. These can encompass cutaneous involvement, such as purpura, peripheral nerve damage causing mononeuritis multiplex, and other neurological symptoms. Gastrointestinal manifestations, although infrequent, can result in complications, such as abdominal pain, gastrointestinal bleeding, and pancreatitis.
Cardiac involvement in EGPA is a significant concern, with manifestations including myocarditis, pericarditis, and coronary artery disease. Pulmonary involvement is common, ranging from infiltrates on imaging to severe necrotizing vasculitis leading to lung tissue destruction.
Renal complications in EGPA can result in glomerulonephritis and renal failure, contributing to the overall disease burden and prognosis. While EGPA can affect multiple organs, its association with the biliary system, specifically the bile ducts and gallbladder, is exceptionally rare, and existing literature and reviews provide limited information on such occurrences.
In this report, we present a unique case of EGPA with concurrent sclerosing cholangitis and cholecystitis, illustrating a rare finding within the context of EGPA. This case emphasizes the importance of considering EGPA in the differential diagnosis when encountering patients with unexplained biliary system involvement and underscores the need for comprehensive evaluation and management of patients with EGPA [1–3].
Case Report
A 47-year-old man with a 20-year history of bronchial asthma, well-controlled with continuous steroid inhalation, presented to our hospital with fatigue and cough persisting for 6 months. A medical examination conducted 3 months earlier had revealed an increased white blood cell (WBC) count (10 000/μL), and a chest radiography showed an infiltrating shadow in the right lung, but a detailed examination had not been conducted. He arrived at our hospital following a slight fever, exacerbation of fatigue for 4 weeks, epigastralgia for 3 weeks, and unintentional weight loss (7 kg in 6 months). Our laboratory test results revealed eosinophilia. A computed tomography (CT) scan of the chest showed central bronchiectasis in both lungs and mucus plugs in the lower lobes of both bronchi. An abdominal CT scan showed thickening of the bile duct and gallbladder walls. Under suspicion of EGPA and sclerosing cholangitis and cholecystitis, the patient was admitted for an endoscopic retrograde cholangiopancreatography (ERCP) and subsequent treatment. He had a history of nasal polyps and sinusitis treats 5 years earlier.
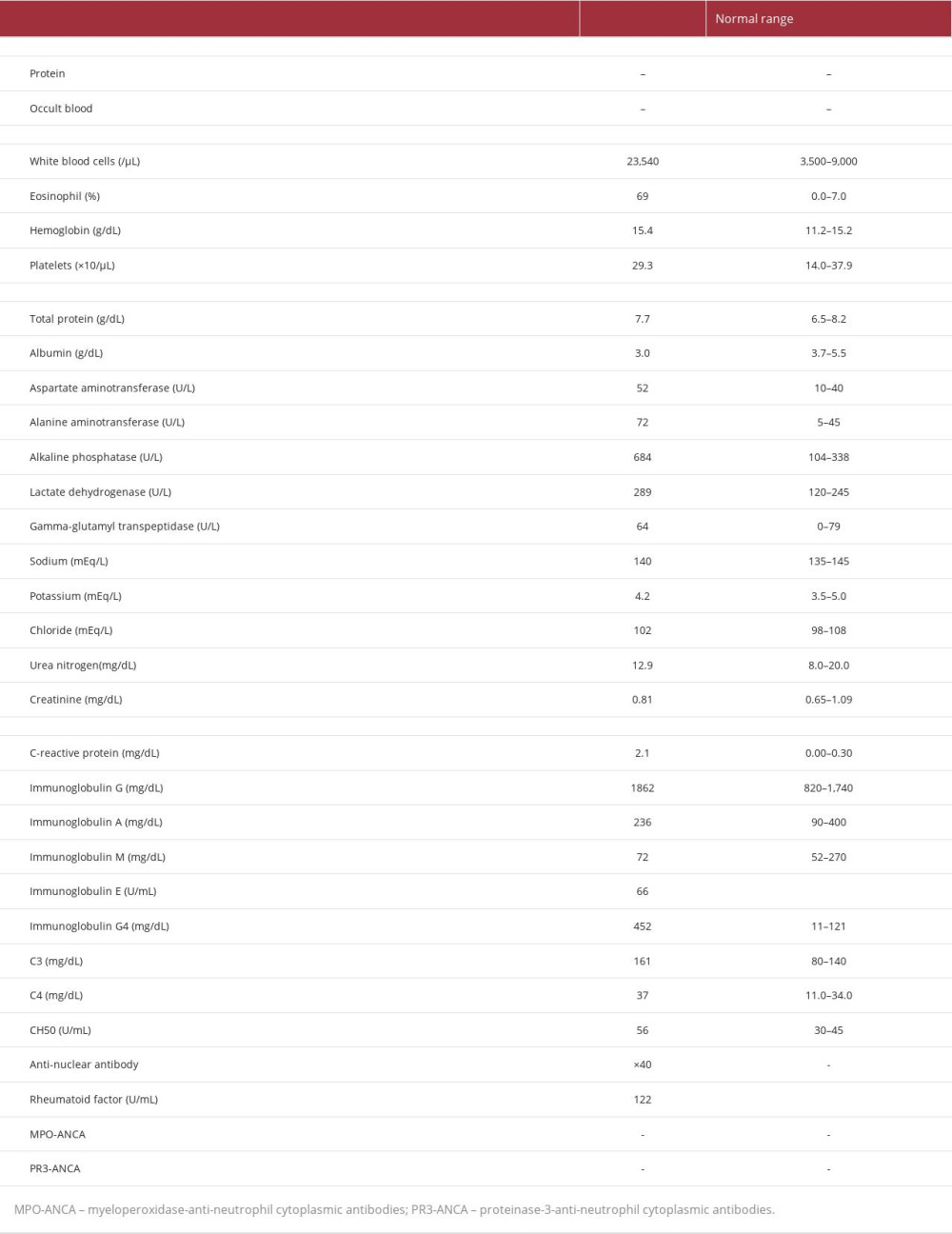
On admission, the patient was medicated with olopatadine to control the symptoms of allergic rhinitis, and with fluticasone furoate and vilanterol trifenatate for inhalation to manage his bronchial asthma. His vital signs on admission were as follows: temperature, 36.3°C; blood pressure, 140/92 mmHg; heart rate, 100 beats per min; respiratory rate, 16 breaths per min; and oxygen saturation, 90% in room air. A physical examination showed tenderness in his upper abdomen and purpura on the upper left arm. However, his respiratory and heart sounds were normal. Moreover, he had no motor or sensory neuropathy. The results of the laboratory tests on admission are presented in Table 1. A bone marrow biopsy revealed no dysplasia and malignant cells. A skin biopsy had initially been considered for the purpura on his arm but was not performed because the purpura disappeared a few days after admission. The genetic analyses showed normal FIP1L1/PDGFRα. A CT scan of the chest showed ground-glass opacities in the peripheral and lower lobes of the right lung, bronchial wall thickening in the lower lobes, and mucus plugs (Figure 1A, 1B). A CT scan of the paranasal sinuses revealed soft tissue densities and mucosal thickening of the nasal mucosa in the left maxillary sinus (Figure 1C, 1D). Abdominal ultrasonography showed a circumferential thickening of the medial layer of the gallbladder wall (Figure 1E). Magnetic resonance cholangiopancreatography led to the suspicion of a smooth narrowing of the common hepatic duct, without gallstones or common bile duct stones (Figure 1F). An abdominal contrast CT scan revealed diffuse wall thickening mainly due to subserosal edema that appeared consecutively throughout the gall-bladder, cystic duct, left and right hepatic ducts, and common bile duct (Figure 1G, 1H). These findings suggested sclerosing cholangitis and cholecystitis. ERCP for biopsy of the thickened bile duct wall was not performed, because of a high risk of hypoxia. The patient was diagnosed with EGPA according to the classification criteria established by the American College of Rheumatology, alone [4], and in association with the European Alliance of Associations for Rheumatology [5].
On the first day of treatment, the patient was administered prednisolone (PDN) 50 mg/day (1 mg×kg−1×day−1). His respiratory symptoms and upper abdominal pain improved for 3 days, hepatobiliary enzymes, WBC, and eosinophil counts returned to normal, and CRP levels became negative (Figure 2). Three weeks after the administration of PDN, azathioprine (AZA) 100 mg/day (2 mg×kg−1×day−1) was added, and the PDN dose was tapered for remission maintenance therapy. He was discharged the same day.
All initial scans were repeated on the 36th day of PDN treatment to verify the improvements that the patient demonstrated. A CT scan of the chest and the sinuses showed an absence of ground-glass opacities in the lung (Figure 3A), no soft tissue density, and no thickening of the mucosa in the sinuses (Figure 3B). Furthermore, abdominal ultrasonography and a contrast-enhanced CT scan showed a reduction of the wall thickening of the gallbladder and bile duct (Figure 3C–3E), and magnetic resonance cholangiopancreatography showed disappearance of the stenosis of the common hepatic duct (Figure 3F). Based on these positive results, the patient was discharged on the same day. Twenty-four months after administration, the PDN dose was tapered to 5 mg/day. The patient showed no relapse during the 2-year follow-up period in our hospital, and his WBC count, eosinophil count, and CRP level remained within the reference ranges.
Discussion
Herein, we describe the case of a 47-year-old man who developed EGPA complicated with sclerosing cholangitis and cholecystitis. ERCP could not be performed owing to the low oxygen levels of the patient and the risk of hypoxia. The clinical course of the patient showed that he had bronchial asthma and eosinophilia with symptoms of vasculitis, leading to the diagnosis of EGPA. Treatment with PDN, and subsequent addition of AZA, led to full remission. The sclerosing cholangitis and cholecystitis responded well to PDN. Eosinophilic cholangitis is a disease histologically characterized by the infiltration of eosinophilic cells into the bile duct, promoting an inflammatory response, and sclerosing cholangitis associated with EGPA, which, in this case, may have been of eosinophilic origin. Disappearance of the abnormal image findings in the bile duct and gallbladder after the administration of PDN suggests that this specific case of EGPA was additionally complicated by concurrent eosinophilic cholangiopathy.
The prognosis and frequency of eosinophilic cholangiopathy with EGPA remain elusive. Previously, only 19 cases of EGPA in conjunction with eosinophilic cholecystitis have been reported [6–23], while only 2 studies reported EGPA together with eosinophilic cholangitis [24,25]. Most of the reported cases had a histopathological diagnosis, but in the present case, the contemporaneous appearance of EGPA and cholecystitis was diagnosed based on the patient’s symptoms, laboratory test results, and imaging findings, without a pathological diagnosis.
EGPA is reportedly associated with increased serum IgG4 levels, bronchial asthma, and eosinophilia, while IgG4-related disease is associated with the same symptoms. The nearly identical features of both the diseases [26–30] make it challenging to differentiate between them. Even though ANCA-associated vasculitis has been reported previously [30], we could not confirm any reports of EGPA combined with IgG4-related disease [31]. In the present case, IgG4-related disease could have been considered a differential disease because of the elevated serum IgG4 levels and the thickening of the bile duct wall and gallbladder wall. However, the diagnosis of EGPA, rather than IgG4-related disease, was made because the patient had a slight fever, felt general malaise, had lost weight, suggesting vasculitis, and elevated CRP levels.
In general, EGPA is mainly treated with PDN; the EGPA practice guidelines recommend the use of PDN or cyclophosphamide for remission induction therapy and AZA for remission maintenance therapy [32]. In a recent case report, eosinophilic cholangitis, in conjunction with EGPA, was successfully treated by combining PDN and mepolizumab [24]. In the present case, PDN was administered in monotherapy, without the addition of cyclophosphamide or mepolizumab, which was sufficient to induce remission, while AZA was added to maintain it. The prescribed dose of PDN was reduced to 5 mg/day and has been kept stable over the past 2 years, without relapse. Therefore, we conclude that EGPA in combination with a suspected eosinophilic cholangiopathy can be treated with PDN alone, without mepolizumab, which can lead to remission. At this time, it is not possible to report on the long-term maintenance of remission or relapse. Thus, careful follow-up remains essential.
Conclusions
To summarize, we report a case of steroid-responsive sclerosing cholangitis and cholecystitis with new-onset EGPA, which went into remission and then maintenance using a combined treatment of PDN and AZA. Questions concerning the established long-term prognosis of eosinophilic cholangiopathy with EGPA remain unanswered. Further studies require the examination of more patients with EGPA and a longer follow-up period.
Figures
References:
1.. Furuta S, Iwamoto T, Nakajima H, Update on eosinophilic granulomatosis with polyangiitis: Allergol Int, 2019; 68; 430-36
2.. Trivioli G, Terrier B, Vaglio A, Eosinophilic granulomatosis with polyangiitis: Understanding the disease and its management: Rheumatology (Oxford), 2020; 59; iii84-94
3.. Chang HC, Chou PC, Lai CY, Tsai HH, Antineutrophil cytoplasmic antibodies and organ-specific manifestations in eosinophilic granulomatosis with polyangiitis: A systematic review and meta-analysis.: J Allergy Clin Immunol Pract, 2021; 9; 445-52.e6
4.. Masi AT, Hunder GG, Lie JT, The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis): Arthritis Rheum, 1990; 33; 1094-100
5.. Grayson PC, Ponte C, Suppiah R, 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis: Ann Rheum Dis, 2022; 81; 309-14
6.. Imai H, Nakamoto Y, Nakajima Y, Allergic granulomatosis and angiitis (Churg-Strauss syndrome) presenting as acute acalculous cholecystitis.: J Rheumatol, 1990; 17; 247-49
7.. Boggi U, Mosca M, Giulianotti PC, Surviving catastrophic gastrointestinal involvement due to Churg-Strauss syndrome: Report of a case.: Hepatogastroenterology, 1997; 44; 1169-71
8.. Kalyoncu A, Karakaya G, Sahin A, Artvinli M, Experience of 10 years with Churg-Strauss syndrome: an accompaniment to or a transition from aspirin-induced asthma?: Allergol Immunopathol (Madr), 2001; 29; 185-90
9.. Nishie M, Tomiyama M, Kamijo M, Acute cholecystitis and duodenitis associated with Churg-Strauss syndrome: Hepatogastroenterology, 2003; 50; 998-1002
10.. Tatsukawa H, Nagano S, Umeno Y, Oribe M, Churg-Strauss syndrome with cholecystitis and renal involvement: Intern Med, 2003; 42; 893-96
11.. Suzuki M, Nabeshima K, Miyazaki M, Churg-Strauss syndrome complicated by colon erosion, acalculous cholecystitis and liver abscesses.: World J Gastroenterol, 2005; 11; 5248-50
12.. Rolla G, Tartaglia N, Motta M, Warning nonrespiratory symptoms in asthma: Catastrophic abdominal involvement in a case of Churg-Strauss syndrome: Ann Allergy Asthma Immunol, 2007; 98; 595-97
13.. Francescutti V, Ellis AK, Bourgeois JM, Ward C, Acute acalculous cholecystitis: An unusual presenting feature of Churg-Strauss vasculitis: Can J Surg, 2008; 51; E129-30
14.. Yüksel I, Ataseven H, Başar O, Churg-Strauss syndrome associated with acalculous cholecystitis and liver involvement: Acta Gastroenterol Belg, 2008; 71; 330-32
15.. Sironen RK, Seppä A, Kosma VM, Kuopio T, Churg-Strauss syndrome manifested by appendicitis, cholecystitis and superficial micronodular liver lesions – an unusual clinicopathological presentation: J Clin Pathol, 2010; 63; 848-50
16.. Lenders G, Goethals M, Verstreken S, Acalculous cholecystitis and tamponade: An unusual combination?: Acta Cardiol, 2011; 66; 383-85
17.. Choi JY, Kim JE, Choi IY, Churg-Strauss syndrome that presented with mediastinal lymphadenopathy and calculous cholecystitis: Korean J Intern Med, 2016; 31; 179-83
18.. Zeng M, Liu X, Liu Y, Eosinophilic granulomatosis with polyangiitis presenting with skin rashes, eosinophilic cholecystitis, and retinal vasculitis: Am J Case Rep, 2016; 17; 864-68
19.. Ye L, Lu X, Xue J, Eosinophilic granulomatosis with polyangiitis complicated by cholecystitis: A case report and review of the literature: Clin Rheumatol, 2016; 35; 259-63
20.. Ohnuki Y, Moriya Y, Yutani S, Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) complicated by perforation of the small intestine and cholecystitis: Intern Med, 2018; 57; 737-40
21.. Ito H, Mishima Y, Cho T, Eosinophilic cholecystitis associated with eosinophilic granulomatosis with polyangiitis: Case Rep Gastroenterol, 2020; 14; 668-74
22.. Hattori K, Teramachi Y, Kobayashi Y, A case of effective mepolizumab induction therapy for severe eosinophilic granulomatosis with polyangiitis diagnosed by eosinophilic cholecystitis and interstitial nephritis.: Case Rep Rheumatol., 2021; 2021; 6678893
23.. Takeda E, Shikino K, Eosinophilic granulomatosis polyangiitis associated with acute acalculous cholecystitis.: BMJ Case Rep., 2021; 14; e243536
24.. Yoshihara R, Komai T, Nagafuchi Y, Eosinophilic cholangitis with eosinophilic granulomatosis with polyangiitis: A case report and review of the literature.: Allergol Int, 2020; 69; 154-56
25.. Franco DL, Ruff K, Mertz L, Eosinophilic granulomatosis with polyangiitis and diffuse gastrointestinal involvement.: Case Rep Gastroenterol, 2014; 8; 329-36
26.. Varghese JL, Fung AWS, Mattman A, Clinical utility of serum IgG4 measurement: Clin Chim Acta, 2020; 506; 228-35
27.. Kanda R, Kubo S, Nakano K, A case of eosinophilic granulomatosis with polyangiitis as a mimicker of IgG4-related disease: Mod Rheumatol Case Rep, 2020; 4; 278-82
28.. Wu Z, Zhang S, Li P, Elevated serum IgG4 was found in eosinophilic granulomatosis with polyangiitis.: J Clin Rheumatol, 2021; 27; e501-4
29.. Zhou L, Cao F, Fan S, A case of eosinophilic granulomatosis with polyangiitis complicated with a similar condition to IgG4 related lung disease: BMC Pulm Med, 2019; 19; 154
30.. Erden A, Bolek EC, Yardimci KG, Do ANCA-associated vasculitides and IgG4-related disease really overlap or not?: Int J Rheum Dis, 2019; 22; 1926-32
31.. Yoo J, Song JJ, Park YB, Lee SW, Definite IgG4-related disease had no overlap with eosinophilic granulomatosis with polyangiitis in Korean patients: A pilot study in one centre: Clin Rheumatol, 2020; 39; 3009-15
32.. Chung SA, Langford CA, Maz M, 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of anti-neutrophil cytoplasmic antibody-associated vasculitis: Arthritis Rheumatol, 2021; 73; 1366-83
Figures
In Press
21 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942921
22 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943346
24 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943560
26 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943893
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250