13 December 2023: Articles 
Unveiling the Rare Complication: Statin-Induced Immune-Mediated Necrotizing Myopathy
Unknown etiology, Challenging differential diagnosis, Rare disease, Adverse events of drug therapy, Educational Purpose (only if useful for a systematic review or synthesis)
Farheen Hussain Chowdhury1BCDEFG*, Olena Mahneva2ABCDEF, Maniekha Maharaj1BE, Werther Marciales1CGDOI: 10.12659/AJCR.941387
Am J Case Rep 2023; 24:e941387
Abstract
BACKGROUND: Statin-induced necrotizing autoimmune myopathy is an exceptionally rare yet severe complication of statin therapy that may develop in individuals at any time during their exposure to statins. The development of proximal muscle weakness, muscle pain, and elevated creatine kinase (CK) levels in patients while taking statins should prompt clinical consideration of statin-induced myopathy. The pathophysiology arises from the production of auto-antibodies, which target the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) enzyme, leading to the aggressive breakdown of myofibrils.
CASE REPORT: Here, we present a case of a 59-year-old woman with a medical history of dyslipidemia who developed anti-HMG-CoA reductase antibodies after taking atorvastatin. She came to the emergency department with complaints of severe proximal muscle weakness. The laboratory workup showed an elevated CK level up to 12 000 IU/L. Despite discontinuing atorvastatin, the patient’s elevated CK levels persisted. The patient underwent a muscle biopsy, demonstrating myofibril necrosis. Serological analysis showed anti-HMG-CoA reductase antibodies in the patient’s serum, which led to the diagnosis of immune-mediated necrotizing myopathy due to statins. The patient’s statin therapy was promptly discontinued, and she was treated with a high dose of IV corticosteroids. After the patient’s discharge, brief discontinuation of the corticosteroids resulted in CK elevation and a return of symptoms. This led to the second re-admission and restarting of corticosteroids until stabilization and discharge.
CONCLUSIONS: This case represents an important reminder for clinicians to recognize the possibility of statin-induced immune-mediated necrotizing myopathy in patients presenting with proximal muscle weakness while taking a statin, notwithstanding the rarity of this condition.
Keywords: myositis, Myotoxicity, Anti-HMG-CoA Reductase-Induced Myopathy, Statin-Induced Myopathy, statins, HMG-CoA Reductase Enzyme, myonecrosis
Background
Statins are among the most widely used pharmacological agents to prevent the progression of cardiovascular diseases such as atherosclerosis, peripheral arterial disease, myocar-dial infarction, and stroke [1]. They work as reversible competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) and lead to a reduction of intracellular synthesis of cholesterol by blocking the conversion of HMG-CoA to mevalonic acid [2]. Further, studies have demonstrated that statins stabilize atherosclerotic plaque and reduce endothelial inflammation [3–5]. Due to their great benefits to human health, they are widely prescribed in the United States and worldwide [4].
Although generally considered safe, some adverse reactions to statins include increased liver transaminases (without major toxicity), transient proteinuria, and microscopic hematuria, or in a pre-operative setting, autoimmune myopathy, myalgias, and diabetes mellitus [6,7]. One of the most severe myotoxic events is rhabdomyolysis, the breakdown of muscle fibers that could lead to acute renal failure and, as seen in our patient, myopathy [7].
Statin-associated muscular symptoms, which include myalgia, weakness, and fatigue, are reported by 10 to 25% of patients taking them [8]. Clinical studies show that the estimated incidence of statin-mediated myopathy is 1.5% to 5% [9]. A statin-induced myotoxicity (SIM) classification was introduced by Alfirevic et al in 2014 [5,10]. This classification system includes 6 classes of myopathies associated with statins. A SIM score of 0–1 demonstrates a mild creatine kinase (CK) elevation and or mild myalgia, which could be related to transient conditions such as exercise and dehydration [10]. A SIM score of 2 presents with severe myalgia that interferes with the patient’s quality of life and prompts statin discontinuation. A SIM score of 3–5 corresponds to cases of variable degrees of muscle necrosis and causes conclusive discontinuation of the therapy, which results in symptom improvement. A SIM score of 6, the most severe class of statin-induced myopathy, presents with severe muscle necrosis and the production of HMG-CoA reductase antibodies. Usually, patients present with proximal bilateral weakness of the upper and lower extremities and more pronounced lower limb weakness [11]. The CK levels are usually higher than 2000 IU/L in these patients [12]. These patients must discontinue statin therapy and should never be reinstated. Some sources estimate the incidence of immune-mediated statin-induced myopathy at about 2 per 1 million patients, while others estimate the incidence at 2–3/100 000 patients taking statins [12,13].
Case Report
HISTORY:
A 59-year-old woman with a history of generalized anxiety disorder, hypertension, morbid obesity, and dyslipidemia presented to the Emergency Room (ER) with chief complaints of muscle weakness and myalgias of 3 to 5 months duration, which started in her legs, making it more difficult for her to walk, maintain balance, as well as rise out of a chair or out of her bed at night to use the bathroom. She said the weakness had progressed to her shoulders and neck and was present throughout the day. Her weakness was associated with extreme fatigue, “brain fogginess,” and cramping in her calves. She denied any trauma, heat or cold intolerance, changes in bowel habits or appetite, unintentional weight loss, headaches, fever, recent illness, sick contacts, changes in vision, dizziness, or loss of consciousness. She described the rapid progression of symptoms during the previous 2 weeks before admission: she had stopped climbing the stairs in her house and could not walk without using a cane, becoming very short of breath, and having sub-sternal chest pressure after walking only 10 feet. She said she could not do simple daily living activities, getting very tired and weak while dressing or cleaning after herself. To investigate the cause of her symptoms, she visited her primary care provider (PCP), who referred her to a neurologist. She was instructed by her neurologist to urgently come to the ER due to her recent blood work results demonstrating CK levels up to 12 000 IU/L (normal reference range: 34–145 IU/L). She was further evaluated and admitted to the hospital.
DIAGNOSIS:
The following list of differential diagnoses was considered in this patient: hypothyroid myopathy, vitamin B deficiency-induced neuropathy, autoimmune myopathies such as myasthenia gravis, myositis associated with rheumatoid arthritis, anti-mitochondrial antibody (AMA)-positive myositis, lupus myositis, scleromyositis, polymyositis, statin-induced immune-mediated myositis, and statin-induced rhabdomyolysis. Physical examination was unremarkable except for weakness of proximal upper extremities, with 3/5 motor strength on bilateral shoulder abduction, and weakness in lower extremities, showing motor strength 3/5 bilaterally with hip flexion. Physical therapy evaluation showed a “6-Clicks” score of 20/24, and the Timed Get-up and Go test was within the normal limit of 5 seconds. Gower’s sign was absent. The sensation and osteotendinous reflexes were intact bilaterally in the upper and lower extremities. The patient’s blood and serum were sent to the laboratory, and she underwent magnetic resonance imaging (MRI) of bilateral femurs without contrast while lab-work was in process (Figure 1). The following findings were described for the left lower proximal extremity: “intramuscular edema involving the left rectus femoris muscle proximally, mild edema at adductor and hamstring musculature.” The following findings were described for the right lower proximal extremity: “mild intramuscular edema involving obturator internus, adductor, and ham-string musculature, heterogeneous areas of intramuscular edema in the right rectus femoris proximally.” Her right thigh-muscle biopsy results showed muscle necrosis consistent with antibody-induced myositis. Her serum results, which were initially sent at the beginning of her admission, were obtained and showed anti-HMG-CoA reductase antibodies detected by an enzyme-linked immunosorbent assay (ELISA), consistent with statin-induced immune-mediated necrotizing myopathy. Antinuclear antibody, rheumatoid factor, anti-striated muscle antibody, anti-scleroderma-70 antibody, anti-centromere B antibody, anti-DNA antibody, and mitochondrial antibody tests were negative.
Since anti-HMG-CoA reductase antibodies may affect the heart, her high-sensitivity troponin I levels were also measured and found to be 5.40 ng/L, 5.14 ng/L, and 3.86 ng/L (normal reference range 0.00–34.11 ng/L). Electrocardiogram (EKG) was unremarkable, with a normal sinus rhythm. Transthoracic echocardiography showed an ejection fraction > 65%, normal diastolic function, and no regional wall motion changes, with the left ventricle being normal in structure and function; the mitral valve demonstrated mild mitral regurgitation and annular calcification. These results did not suggest cardiac involvement in this patient.
To demonstrate whether statin toxicity had any effects on kidney and liver function, alkaline phosphatase, aspartate transaminase (AST), and alanine aminotransferase (ALT) were obtained and are shown in Table 1. The same table also shows the patient’s CK values and blood glucose levels. The creatinine was in the lower-than-normal range except on days 7, 12, and 13, indicating that there was not a problem with creatinine excretion. Alkaline phosphatase levels were within the normal range. AST and ALT were elevated at the presentation and stayed high throughout the patient’s hospital stay. Her inpatient treatment led to a downtrend in liver enzymes. However, the temporary discontinuation of corticosteroids on day 11 resulted in a rise in liver enzymes. There were no hepatic symptoms. Surprisingly, on day 8, there was a transient elevation of CK, creatinine, alkaline phosphatase, AST, ALT, and blood glucose. A transiently elevated blood glucose level (231 mg/dL) on day 8 may have resulted in a potential dehydration effect, leading to higher-than-expected values of other measured metabolites.
The patient’s complete blood counts were also determined, and the values are shown in Table 2. Elevated white blood cells were possibly a result of corticosteroid-induced reactive leukocytosis. Furthermore, on day 8, there was an unexpected elevation of white blood cells, red blood cells, platelets, hemoglobin concentration, and hematocrit. This may have resulted from dehydration due to hyperglycemia, as demonstrated by elevated glucose levels on day 8 in Table 1. Hyperglycemia may have resulted from the high-dose IV methylprednisolone therapy described in the Treatment section. Additional blood tests performed were hemoglobin A1C percentage, C-reactive protein levels (CRP), erythrocyte sedimentation rate, thyroid function tests (thyroid stimulating hormone and free T4 levels), and vitamin B12 levels. The results are shown in Table 3. Normal thyroid function tests and vitamin B12 levels initially helped to rule out hypothyroidism and B12 deficiency, which can cause myopathy and balance problems, respectively. The only abnormal value was CRP at 4.69 mg/dL, indicative of an active autoimmune condition (levels between 1 and 10 mg/dL).
TREATMENT:
The patient was started on high-dose IV methylprednisolone (Solu-Medrol) 1 gram per day for a total of 10 days until she was discharged. She was also continued on her home medications: lisinopril for hypertension and alprazolam for anxiety disorder. She was extensively counseled on the diagnosis and strongly advised to avoid future statin therapy. The patient was discharged on 70 mg prednisone daily as a 10 mg/5 mL oral liquid formulation until followup with her primary provider or the neurologist. On the day of the discharge, her CK levels were recorded to be 2304 IU/L, and her muscle weakness and pain had also substantially improved.
FURTHER FOLLOWUP:
Due to logistical issues, such as the unavailability of the prescribed dosage and formulation, the patient’s pharmacy could not fill the prescription of 70 mg prednisone upon the initial hospital discharge. As expected, the patient’s muscle fatigue and pain symptoms rapidly escalated to the point that she felt too weak to walk and had to be re-admitted 36 hours later. Her CK levels increased from 2304 IU/L from the day of initial discharge to 3190 IU/L on the day of re-admission, and after initiating IV methylprednisolone (Solu-Medrol) therapy again, decreased to 1759 U/L. Upon repeat CK level stabilization, the patient was discharged a second time without further re-admission but given instructions to continue with followup and refill her steroid medication of 70 mg prednisone by combining 50 mg and 20 mg pill formulations together. Her steroid taper plan was deferred to her PCP and the neurologist.
Discussion
Patients with statin-induced immune-mediated necrotizing myopathy may present with delayed symptoms years after the initial statin exposure. The mechanism of how statins induce antibody generation is presently unknown. Our patient’s symptoms started between 4.5 to 5 years after initiation of the statin therapy. From a systematic review of 100 cases, the mean duration of statin therapy before symptom onset was 40.48 months [14]. Similarly, a cohort study of patients demonstrated a mean of 38.8 months (range 15–84 months) from the atorvastatin exposure until the first CK elevation [15]. There is a published case in which a patient was diagnosed with immune-mediated necrotizing myopathy after taking a statin for 10 years [16]. From this, the times between exposure and symptom onset vary greatly.
Muscle biopsy is the gold standard for diagnosing necrotizing myopathies, but it should be followed by specific antibody serological testing. A positive anti-HMG-CoA reductase antibody ELISA, which could represent a false-positive result, must be confirmed by positive immunoblotting [12,17]. Since the presence of anti-HMG-CoA reductase antibodies is a very specific finding (99.3%), there is a question of whether sero-logical testing, which is quick and non-invasive, should be a preferred method for diagnosis of this type of myopathy in the background of the proximal muscle weakness even after discontinuing the statin therapy [12].
There are reports describing cardiac involvement in anti-HMG-CoA reductase antibody-positive myopathies. These may present as acute heart failure with a decreased systolic function where coronary infarction has been ruled out through invasive testing [18–20]. Therefore, a cardiac screen with an EKG, troponin levels, and echocardiography is warranted in patients with the suspected disease. The patient’s kidney and liver function test results were not unexpected. Previous clinical reports describing laboratory values in patients with immune-mediated statin-induced necrotizing myopathy showed no decrease in kidney function (which would be reflected by high creatinine), no effect on alkaline phosphatase, but elevated liver enzymes, as observed in our patient [21,22].
The management of the patient’s diagnosis is a high dose of IV methylprednisolone (Solu-Medrol), which is considered the first-line treatment for this condition [23,24]. From the literature, a typical starting dose of prednisone (or equivalent) is 1 mg/kg/day [25]. Still, high doses of methylprednisolone are to be considered in patients with severe muscle deficits, as observed in our patient. One gram of IV methylprednisolone is a well-known dose used in flares of immune-mediated diseases such as rheumatoid arthritis, dermatomyositis, polymyositis, and other inflammatory myopathies [26–29]. It is critical for the patient to continue this regimen upon discharge from the hospital and into outpatient followup, but eventual steroid tapering or low-dose maintenance must be considered due to possible steroid therapy complications [13]. In our case, the patient was not able to obtain the corticosteroid from her pharmacy after the hospital discharge, and her symptoms predictably returned, leading to the patient’s re-hospitalization and restarting of inpatient corticosteroid therapy. Interestingly, upon the drastic discontinuation of the corticosteroid therapy, the patient’s CK levels increased beyond the levels on the date of the discharge. This further emphasizes the fact that statin discontinuation alone does not stop the continuous progressive damage to the myofibrils and that pharmacological immune suppression is required for symptom alleviation. It is essential for the patients to follow up with a rheumatologist or a neurologist to develop a long-term pharmacological treatment plan. Such a plan could include the addition of azathioprine at a dose of 3 mg/kg body weight or methotrexate 20–25 mg per week orally or subcutaneously [23]. Besides the above immune-suppressing drugs, rituximab may be considered in combination with methotrexate, though there have been reports that rituximab is ineffective in the treatment of HMG-CoA reductase antibody-positive myopathy [11,23,30,31]. In patients with severe or refractory disease (persistence of symptoms beyond 6 months of steroid treatment), IV immunoglobulin has been reported to be highly effective [11,23,32–36].
In most cases of statin-induced immune-mediated myopathy, the muscular symptoms resolve upon prolonged treatment, and generally, the prognosis is good. In rare instances, the muscle weakness persists beyond years of aggressive treatment [23,37,38]. One of the most feared and commonly reported complications of untreated statin-induced immune-mediated myopathy is dysphagia caused by dysfunction of muscles related to swallowing, difficulty with aspiration, and possibly death. It is of importance to note that due to the rarity of the disease and lack of extensive clinical research that would compare different management modalities, this leaves the choice of treatment to the preference of the physician.
Of note, even when statin therapy is recommended as the first line for primary and secondary prevention of cardiovascular disease and treatment of dyslipidemia, stopping statins should not preclude lipid-lowering therapy [39]. Thus, it is important to evaluate other options to prevent atherosclerosis-related morbidity and mortality in patients with HMG-CoA reductase antibody-positive myopathy. Such alternative medications include ezetimibe, bempedoic acid, and proprotein convertase subtilisin/kexin type 9 inhibitors like inclisiran [40].
Conclusions
In conclusion, statin-induced immune-mediated myopathy is a rare condition, and it is important for clinicians to consider it as a differential diagnosis when a patient presents with a progressive proximal weakness on statin therapy. Timely diagnosis and treatment are imperative to prevent further muscle degeneration, restore a patient’s quality of life, and prevent complications-related mortality. Various cases have been described in the literature, and the present report adds to the information imperative to a better understanding of the disease, since clinical research on statin-induced immune-mediated necrotizing myopathy is scarce. Besides treatment modalities and their successes, information on statin types, dosages, lengths of exposure before symptom onset and diagnosis, and treatment challenges, such as abrupt corticosteroid discontinuation followed by a drastic CK elevation within days of steroid discontinuation, are essential to future systematic analyses and our knowledge on this topic.
Tables
Table 1.. Creatine kinase, kidney and liver function enzymes, and blood glucose levels. The values were measured on different days of the patient’s hospitalization (days 1–13 of hospitalization, D1–13). The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). Kidney function is represented by creatinine levels (Cr); liver function is represented by levels of the following enzymes: alkaline phosphatase (AP), aspartate transferase (AST), and alanine transaminase (ALT). Note: not every test was performed during each day of hospitalization. Creatine kinase (CK) values decreased with corticosteroid treatment and increased upon discontinuation. Kidney function was not reduced during the hospital stay. Liver function enzymes (AP, AST, and ALT) were elevated during the hospitalization; a reduction in the enzyme levels with corticosteroid treatment (D1 through D10) and an increase upon discontinuation was noted (D10 vs D12). The highest blood glucose level was noted on D8.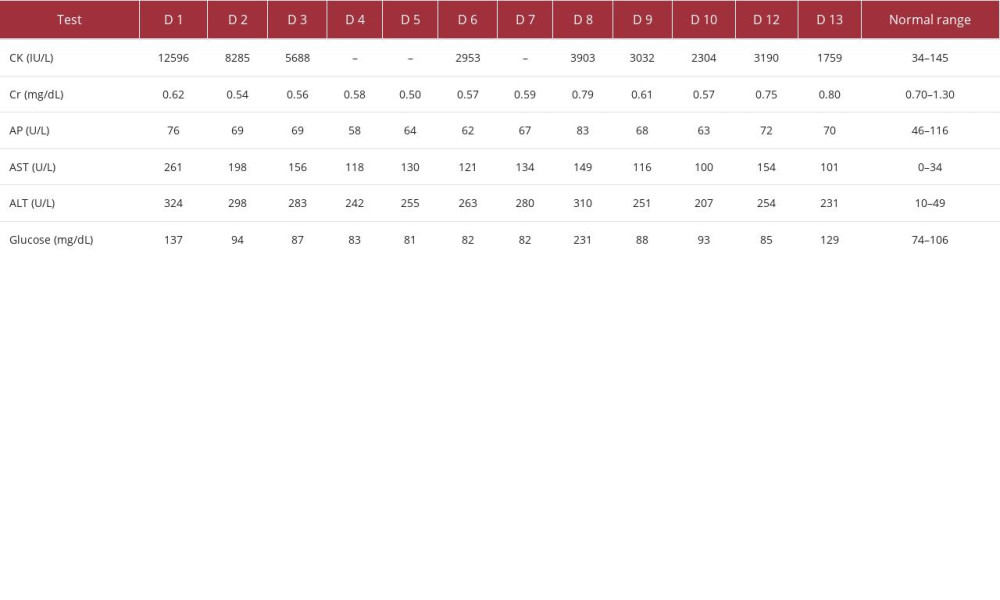 Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy.
Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy.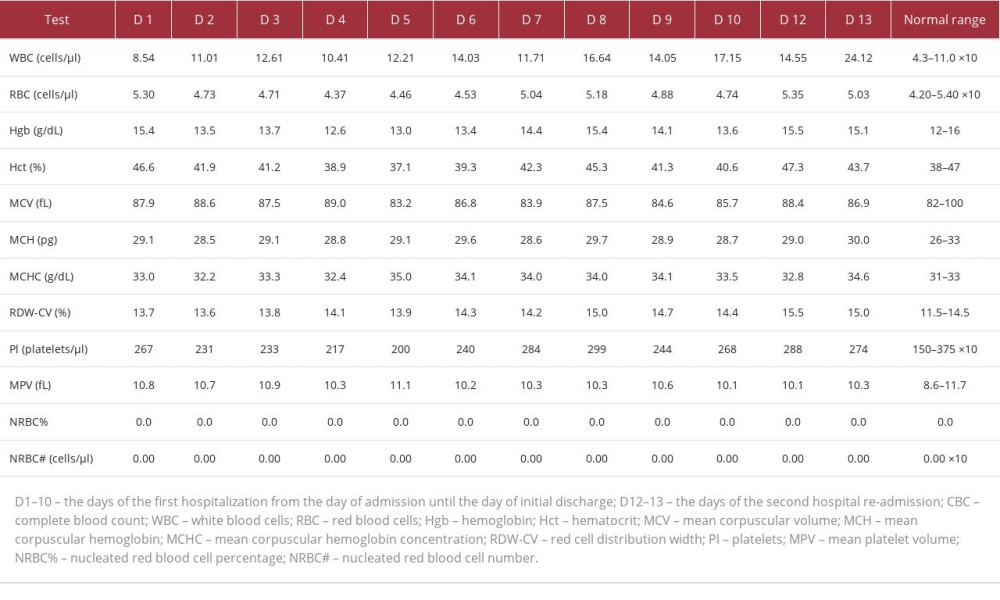 Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process.
Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process.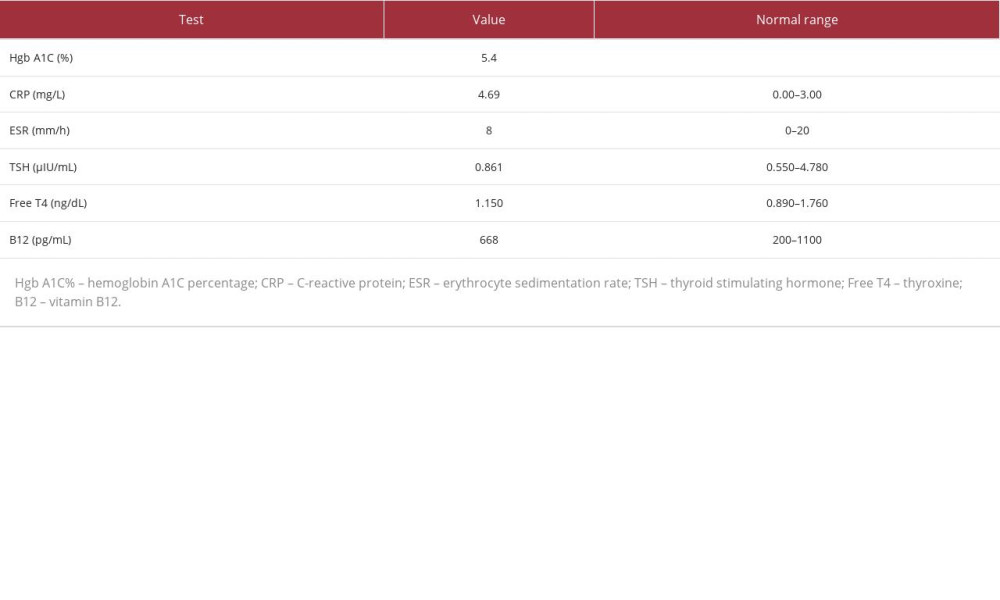
References:
1.. Kumbhani DJ, Steg PG, Cannon CP, Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry: Eur Heart J, 2014; 35(41); 2864-72
2.. Istvan ES, Deisenhofer J, Structural mechanism for statin inhibition of HMGCoA reductase: Science, 2001; 292(5519); 1160-64
3.. Sun W, Lee TS, Zhu M, Statins activate AMP-activated protein kinase in vitro and in vivo: Circulation, 2006; 114(24); 2655-62
4.. Diamantis E, Kyriakos G, Quiles-Sanchez LV, The anti-inflammatory effects of statins on coronary artery disease: An updated review of the literature: Curr Cardiol Rev, 2017; 13(3); 209-16
5.. Du Souich P, Roederer G, Dufour R, Myotoxicity of statins: Mechanism of action: Pharmacol Ther, 2017; 175; 1-16
6.. Newman CB, Preiss D, Tobert JA, Statin safety and associated adverse events: a scientific statement from the American Heart Association: Arterioscler Thromb Vasc Biol, 2019; 39(2); e38-e81
7.. Bełtowski J, Wójcicka G, Jamroz-Wiśniewska A, Adverse effects of statins – mechanisms and consequences: Curr Drug Saf, 2009; 4(3); 209-28
8.. Vinci P, Panizon E, Tosoni LM, Statin-associated myopathy: Emphasis on mechanisms and targeted therapy: Int J Mol Sci, 2021; 22(21); 11687
9.. Ward NC, Watts GF, Eckel RH, Statin toxicity: Circ Res, 2019; 124(2); 328-50
10.. Alfirevic A, Neely D, Armitage J, Phenotype standardization for statin-induced myotoxicity: Clin Pharmacol Ther, 2014; 96(4); 470-76
11.. Kurashige T, Anti-HMGCR myopathy: clinical and histopathological features, and prognosis: Curr Opin Rheumatol, 2021; 33(6); 554-62
12.. Mammen AL, Statin-associated autoimmune myopathy: N Engl J Med, 2016; 374(7); 664-69
13.. Mohassel P, Mammen AL, Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies: Muscle Nerve, 2013; 48(4); 477-83
14.. Nazir S, Lohani S, Tachamo N, Statin-associated autoimmune myopathy: A systematic review of 100 cases: J Clin Rheumatol, 2017; 23(3); 149-54
15.. Troyanov Y, Landon-Cardinal O, Fritzler MJ, Atorvastatin-induced necrotizing autoimmune myositis: An emerging dominant entity in patients with autoimmune myositis presenting with a pure polymyositis phenotype: Medicine (Baltimore), 2017; 96(3); e5694
16.. Güngör C, Wieshmann UC, Severe statin-induced autoimmune myopathy successfully treated with intravenous immunoglobulin: BMJ Case Rep, 2020; 13(5); e234805
17.. Selva-O’Callaghan A, Alvarado-Cardenas M, Marin A, Pinal-Fernandez I, Statins and myositis: The role of anti-HMGCR antibodies: Expert Rev Clin Immunol, 2015; 11(12); 1277-79
18.. Wegner F, Biedermann S, Hudowenz O, Anti-HMGCR myopathy with cardiac involvement: Eur Heart J Cardiovasc Imaging, 2021; 22(12); e166
19.. Pitlick M, Ernste F, Anti-HMGCR myopathy presenting with acute systolic heart failure: BMJ Case Rep, 2019; 12(5); e230213
20.. Ghannam M, Manousakis G, Case report: Immune mediated necrotizing myopathy with IgG antibodies to 3-hydroxy-3-methylglutaryl-coenzyme a reductase (HMGCR) may present with acute systolic heart failure: Front Neurol, 2020; 11; 571716
21.. Stroie OP, Boster J, Surry L, Statin-induced immune-mediated necrotizing myopathy: an increasingly recognized inflammatory myopathy: Cureus, 2020; 12(5); e7963
22.. Dixit A, Abrudescu A, A case of atorvastatin-associated necrotizing autoimmune myopathy, mimicking idiopathic polymyositis: Case Rep Rheumatol, 2018; 2018; 5931046
23.. Abusharar SP, Moku P, Banks S, Immune mediated necrotizing myopathy: A rare complication of statin therapy: Clin Pract, 2020; 10(2); 1248
24.. Rademacher JG, Glaubitz S, Zechel S, Treatment and outcomes in anti-HMG-CoA reductase-associated immune-mediated necrotising myopathy. Comparative analysis of a single-centre cohort and published data: Clin Exp Rheumatol, 2022; 40(2); 320-28
25.. Weeding E, Tiniakou E, Therapeutic management of immune-mediated necrotizing myositis: Curr Treatm Opt Rheumatol, 2021; 7(2); 150-60
26.. Kim KN, Treatment of juvenile rheumatoid arthritis: Korean J Pediatr, 2010; 53(11); 936-41
27.. Hansen TM, Kryger P, Elling H, Double blind placebo controlled trial of pulse treatment with methylprednisolone combined with disease modifying drugs in rheumatoid arthritis: BMJ, 1990; 301(6746); 268-70
28.. Romicka AM, [Use of intravenous megadoses of methylprednisolone for treatment of dermatomyositis in children.]: Pediatr Pol, 1995; 70(3); 243-48 [in Polish]
29.. Cordeiro AC, Isenberg DA, Treatment of inflammatory myopathies: Postgrad Med J, 2006; 82(969); 417-24
30.. Joudeh AI, Albuni MK, Hassen SS, A case report of statin-induced immune-mediated necrotizing myopathy treatment challenges: Case Rep Rheumatol, 2022; 2022; 4647227
31.. Yeo CH, Yaakub A, Wang MCL, Refractory statin-induced immune-mediated necrotizing myositis: Challenges and perils in its management: Cureus, 2022; 14(5); e24778
32.. Oddis CV, Myopathy for the general internist: Statins and much more: Cleve Clin J Med, 2019; 86(10); 656-64
33.. Saleh Y, Herzallah K, Hassanein M, Chang HT, Statin-induced necrotizing autoimmune myopathy: An uncommon complication of a commonly used medication: J Saudi Heart Assoc, 2019; 31(4); 269-72
34.. Joudeh AI, Albuni MK, Hassen SS, A case report of statin-induced immune-mediated necrotizing myopathy treatment challenges: Case Rep Rheumatol, 2022; 2022; 4647227
35.. Stroie OP, Boster J, Surry L, Statin-induced immune-mediated necrotizing myopathy: An increasingly recognized inflammatory myopathy: Cureus, 2020; 12(5); e7963
36.. Nichols L, Pfeifer K, Mammen AL, An unusual case of statin-induced myopathy: Anti-HMGCoA necrotizing autoimmune myopathy: J Gen Intern Med, 2015; 30(12); 1879-83
37.. Chaudhry H, Lin J, Atefi R, A tough pill to swallow: Two cases of statin-induced necrotizing autoimmune myopathy manifesting as dysphagia and transaminitis: SAGE Open Med Case Rep, 2023; 11; 2050313 X221150583
38.. Pulipati P, Reddy JK, Husain SA, Severe anti-HMG-CoAR necrotizing autoimmune myopathy secondary to statin use: Case Rep Rheumatol, 2022; 2022; 6120424
39.. Ferraro RA, Leucker T, Martin SS, Contemporary management of dyslipidemia: Drugs, 2022; 82(5); 559-76
40.. Bardolia C, Amin NS, Turgeon J, Emerging non-statin treatment options for lowering low-density lipoprotein cholesterol: Front Cardiovasc Med, 2021; 8; 789931
Tables
 Table 1.. Creatine kinase, kidney and liver function enzymes, and blood glucose levels. The values were measured on different days of the patient’s hospitalization (days 1–13 of hospitalization, D1–13). The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). Kidney function is represented by creatinine levels (Cr); liver function is represented by levels of the following enzymes: alkaline phosphatase (AP), aspartate transferase (AST), and alanine transaminase (ALT). Note: not every test was performed during each day of hospitalization. Creatine kinase (CK) values decreased with corticosteroid treatment and increased upon discontinuation. Kidney function was not reduced during the hospital stay. Liver function enzymes (AP, AST, and ALT) were elevated during the hospitalization; a reduction in the enzyme levels with corticosteroid treatment (D1 through D10) and an increase upon discontinuation was noted (D10 vs D12). The highest blood glucose level was noted on D8.
Table 1.. Creatine kinase, kidney and liver function enzymes, and blood glucose levels. The values were measured on different days of the patient’s hospitalization (days 1–13 of hospitalization, D1–13). The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). Kidney function is represented by creatinine levels (Cr); liver function is represented by levels of the following enzymes: alkaline phosphatase (AP), aspartate transferase (AST), and alanine transaminase (ALT). Note: not every test was performed during each day of hospitalization. Creatine kinase (CK) values decreased with corticosteroid treatment and increased upon discontinuation. Kidney function was not reduced during the hospital stay. Liver function enzymes (AP, AST, and ALT) were elevated during the hospitalization; a reduction in the enzyme levels with corticosteroid treatment (D1 through D10) and an increase upon discontinuation was noted (D10 vs D12). The highest blood glucose level was noted on D8. Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy.
Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy. Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process.
Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process. Table 1.. Creatine kinase, kidney and liver function enzymes, and blood glucose levels. The values were measured on different days of the patient’s hospitalization (days 1–13 of hospitalization, D1–13). The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). Kidney function is represented by creatinine levels (Cr); liver function is represented by levels of the following enzymes: alkaline phosphatase (AP), aspartate transferase (AST), and alanine transaminase (ALT). Note: not every test was performed during each day of hospitalization. Creatine kinase (CK) values decreased with corticosteroid treatment and increased upon discontinuation. Kidney function was not reduced during the hospital stay. Liver function enzymes (AP, AST, and ALT) were elevated during the hospitalization; a reduction in the enzyme levels with corticosteroid treatment (D1 through D10) and an increase upon discontinuation was noted (D10 vs D12). The highest blood glucose level was noted on D8.
Table 1.. Creatine kinase, kidney and liver function enzymes, and blood glucose levels. The values were measured on different days of the patient’s hospitalization (days 1–13 of hospitalization, D1–13). The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). Kidney function is represented by creatinine levels (Cr); liver function is represented by levels of the following enzymes: alkaline phosphatase (AP), aspartate transferase (AST), and alanine transaminase (ALT). Note: not every test was performed during each day of hospitalization. Creatine kinase (CK) values decreased with corticosteroid treatment and increased upon discontinuation. Kidney function was not reduced during the hospital stay. Liver function enzymes (AP, AST, and ALT) were elevated during the hospitalization; a reduction in the enzyme levels with corticosteroid treatment (D1 through D10) and an increase upon discontinuation was noted (D10 vs D12). The highest blood glucose level was noted on D8. Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy.
Table 2.. Complete blood counts. The CBC values were determined on different days of the patient’s hospitalization. The measurements were done throughout the first hospitalization (D1–10) and the second hospitalization (D12–13). WBC elevation starting on D2 may indicate reactive leukocytosis due to corticosteroid therapy. Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process.
Table 3.. Hemoglobin A1C percentage, C-reactive protein, erythrocyte sedimentation rate, thyroid stimulating hormone, free thyroxine (T4), and vitamin B12 levels. The table shows the values of tests performed to examine the patient’s diabetes status, determine the body’s inflammatory state (CRP, ESR), and rule out secondary causes of muscle weakness and imbalance (hypothyroidism and low vitamin B12 levels). The patient’s test results were normal except for CRP, with values indicative of an autoimmune process. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








