13 November 2023: Articles 
Managing Inflammatory Myofibroblastic Tumor of the Uterus: A Case Report and Comprehensive Review of Pathological and Therapeutic Approaches
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease
Xuechao Ji12ABE, Peiling Zhai1ABE, Hui Wang1CDF, Hanchao Yang3CF, Xinbo Wang1FG*DOI: 10.12659/AJCR.941519
Am J Case Rep 2023; 24:e941519
Abstract
BACKGROUND: Inflammatory myofibroblastic tumor (IMT) is a rare disease, and uterine IMT is even rarer. IMT is hard to distinguish from endometrial polyp and submucous myoma. The treatment of IMT is still controversial. Here, we report a case of uterine IMT, discussing both pathological and therapeutic aspects.
CASE REPORT: A 32-year-old woman was admitted to our hospital for a uterine mass, hypermenorrhea, and anemia. She had been suffering from these symptoms for almost a year. Pelvic ultrasound and MRI revealed a mass about 7 cm in diameter at the bottom of the uterus. Serum tumor markers were negative. She was diagnosed with submucous fibroids of the uterus. Then she underwent hysteroscopic mass resection. Histopathological and immunohistochemistry stain analysis revealed IMT of the uterus. Due to the malignant potential of IMT, she was advised to undergo a total hysterectomy, but she refused because she wanted to retain the uterus and fertility. A watch-and-wait strategy without any therapy was chosen, and the patient is currently disease-free after 18-month follow-up.
CONCLUSIONS: IMT is a disease with malignant potential and may recur at a late stage; hence, a correct diagnosis is essential for patients with IMT. Surgery is the preferred treatment for IMT. For early-stage, young women who want to preserve fertility, conservative surgery is acceptable, but close follow-up is required to avoid recurrence and metastasis. If a patient cannot undergo surgery or the disease has metastasized extensively, targeted therapy for ALK gene, immunotherapy, and other methods can be considered.
Keywords: ACTA2 Protein, Human, Anaplastic Lymphoma Kinase, Granuloma, Plasma Cell, Uterus
Background
An inflammatory myofibroblastic tumor (IMT) is a mesenchymal tumor with extremely low incidence. It mostly occurs in the lung, pelvic, and retroperitoneal soft tissues but rarely in the reproductive system. IMT was previously considered a benign disease; however, it is now recognized to have a risk of local recurrence and distant metastasis. Here, we report a case of uterine IMT.
Case Report
A 32-year-old woman was admitted to our hospital because of a uterine mass, hypermenorrhea, and anemia. She has been pregnant once, given birth once, and now uses condoms for contraception. She has a regular menstrual cycle without tobacco smoking, alcohol use, or substance abuse history. Pelvic ultrasonography revealed a solid echo with a size of 5.1×3.6×3.4 cm in the lower uterine cavity, with a clear boundary, an irregular shape, and rich blood flow signals (Figure 1A, 1B). A biannual pelvic examination revealed that the uterus was at approximately 8 weeks of pregnancy, with medium texture and no tenderness. A pelvic magnetic resonance imaging (MRI) scan demonstrated that a mass of slightly short T1 and long T2 signal was visible at the bottom of the uterine cavity, the shape of the mass was irregular, and the mass size was about 6.0×3.4×4.0 cm with slightly diffusion-weighted imaging with background suppression (DWIBS) signal densification. No enlarged lymph nodes were found on pelvic MRI (Figure 2A–2C). Routine blood testing revealed hemoglobin (Hb) 7.9 mg/dL. Serum tumor markers such as carbohydrate antigen 125, carbohydrate antigen 19-9, and squamous cell carcinoma antigen were negative. Her cervical HPV screening result was normal. These findings suggested a clinical diagnosis of uterine submucous myoma. A hysteroscopic mass resection was performed. Internal examination of the uterine cavity revealed a fibroid with a maximum diameter of 6 cm originating from the anterior wall of the uterine fundus, with a broad pedicle and soft texture. Histopathological examination of the surgical specimens revealed myxoid degeneration in the local area of the tumor, with most tumor cells arranged in bundles accompanied by scattered lymphocyte infiltration (Figure 3A). Immunohistochemical staining demonstrated positive immunoreactivity for smooth-muscle actin (Figure 3B), anaplastic (ALK) (Figure 3C), focal CD10 positivity, and a Ki-67 proliferation index of 5%. Conversely, h-caldesmon, CD117, PHH3, SOX10, and STAT-6 were found to be negative by immunohistochemistry. The final pathological diagnosis was an inflammatory tumor (IMT) of the uterus. The patient was advised to undergo a hysterectomy. However, the patient strongly desired to retain the uterus and fertility and refused a hysterectomy. A watch-and-wait strategy was used without any therapy. The patient’s tumor had no progression after 18-month follow-up, as confirmed by clinical examination and pelvic ultrasound. Table 1 shows the case timeline.
Discussion
IMT is an uncommon mesenchymal tumor with an intermediate malignant potential [1]. It was first discovered by Brunn in 1939 [2]. IMT has a predilection for the lung, mesentery, omentum, and retroperitoneum in children, adolescents, and young adults, and this neoplasm may be ubiquitous [1,3,4]. IMT in the female reproductive tract is rare. The most common site of IMT in the female genital tract reported in the current literature is the uterus [5]. Only 130 cases of uterine IMT have been reported so far. The age of patients with uterine IMT ranged from 3.5 to 78 years, with a median age of 39 years. The tumor was found to be located in the corpus uteri in 60% of cases, associated with pregnancy or placenta in about 30%, and had a cervical origin in about 8% of cases [5,6].
IMT was initially described as an inflammatory pseudotumor of lung tissue and has been referred to by other names, including plasmacytic granuloma, xanthopseudotumor, and pseudosarcomatous myofibroblastic hyperplasia. Initially, IMT was considered a reactive hyperplasia of some benign diseases. Later, it was reported that IMT has the potential for recurrence and distant metastasis. In 2013, the World Health Organization (WHO) classified IMT as bone and soft-tissue tumors, and the risk of local recurrence and distant metastasis was approximately 25% and 2%, respectively [1,7]. The pathogenesis of IMT remains unknown. Recently, with the development of genetic testing technology, it has been confirmed that about 50% of IMT have ALK rearrangement on chromosome 2p23, which leads to the fusion of the 3’-terminus kinase coding part of ALK with the 5’-terminus of the chaperone gene, resulting in the strong influence of the 5’-terminus promoter of the kinase domain of IMT. ALK protein expression occurs [7].
IMT was found incidentally in 54% of cases. In nearly 30% of cases, abnormal uterine bleeding is the main symptom. In a very small number of cases (3%), an inflammatory syndrome has been reported as the dominant presentation before diagnosis [4,8–12]. CT and MRI findings of IMT are soft-tissue masses with ill-defined boundaries and heterogeneous enhancement, with or without invasion of adjacent structures [13]. IMT generally does not exhibit specific hematological changes. Some patients have thrombocytosis, an elevated erythrocyte sedimentation rate, and elevated C-reactive protein levels [3,5]. In this case, the patient’s hemoglobin was low due to hypermenorrhea.
Histologically, IMT comprises proliferating spindle fibroblasts and myofibroblasts, surrounded by lymphocytes and plasma cells. Spindle cells usually have mild atypia but no mitotic figures [3,4]. IMT is driven by ALK rearrangements involving various fusion gene chromosomes, and 50% to 60% of cases show corresponding ALK receptor tyrosine kinase staining, leading to its aggressive features, such as increased local recurrence after resection and distant metastasis [14–16].
Immunohistochemical staining demonstrated that the positive rates of vimentin, SMA, and muscle-specific actin were 99%, 92%, and 89%, respectively [6,17]. Myofibroblastic spindle cells in IMT cells are considered to be important clinical markers of myogenic immunoreactivity that can be expressed as smooth bodies such as non-positive SMA. Therefore, non-negative or positive SMA immunoreactivity is an essential marker for early clinical diagnosis [4,17]. In this case, immunohistochemical staining of the tumor was positive for both ALK and SMA. The tumor was confined to the uterus and had not metastasized, which is consistent with previous reports.
There is no consensus on the treatment of uterine IMT. Given the malignant potential of this tumor, complete surgical re-section may be the best treatment option [10,11]. For patients who cannot undergo surgery or who have recurrences and metastases, targeted therapy with tyrosine kinase inhibitors (eg, crizotinib and ceritinib) may have certain therapeutic effects if genetic testing shows ALK rearrangement [18,19]. Some researchers reported that non-steroidal anti-inflammatory drugs, anti-tumor necrosis factor antibodies, and corticosteroids could reduce the tumor volume and the difficulty of surgery [20–23]. It has been reported that in 70% of ALK-negative IMT with metastasis and/or recurrence, the expression of programmed death ligand 1 (PD-L1) was elevated. Immune checkpoint inhibitors may play a role in patients with an advanced ALK-negative IMT [24]. CD30 is a potential therapeutic target for epithelioid inflammatory myofibroblastic sarcomas. Brentuximab vedotin (BV) is a monoclonal CD-30 antibody used in treating lymphoma and may help treat recurrent and metastatic IMT [25]. Chemotherapy is mainly used in the pediatric population. These chemotherapy regimens mainly include methotrexate-vinblastine, followed by ifosfamide-doxorubicin, ifosfamide alone, or carboplatin-paclitaxel [26,27].
Conclusions
IMT is rare in clinical practice. To avoid misdiagnosis as a fully malignant or benign tumor, gynecologists should be aware that IMT can occur in the female reproductive tract. Accurate diagnosis and complete surgical resection are crucial. Methods such as targeted therapy, immunotherapy, and chemotherapy can be considered for patients who cannot be surgically treated.
Figures
Tables
Table 1.. Timeline.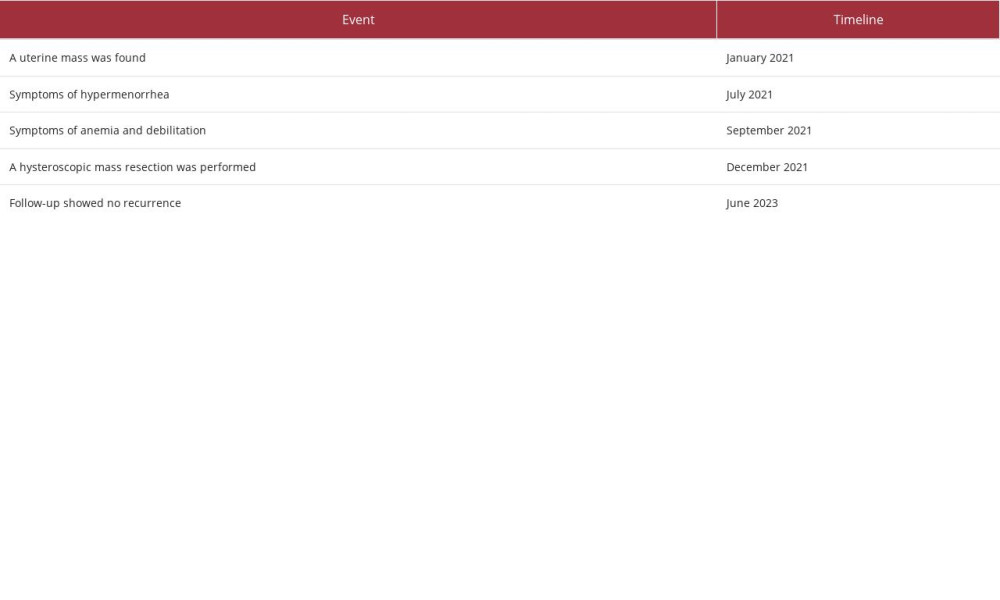
References:
1.. Coindre JM, New WHO classification of tumours of soft tissue and bone: Ann Pathol, 2012; 32(5 Suppl.); S115-16
2.. Al Sannaa G, Wimmer JL, Ayala AG, Ro JY, An isolated inflammatory myofibroblastic tumor of adrenal gland: Ann Diagn Pathol, 2016; 25; 33-36
3.. Gleason BC, Hornick JL, Inflammatory myofibroblastic tumours: Where are we now?: J Clin Pathol, 2008; 61(4); 428-37
4.. Coffin CM, Watterson J, Priest JR, Dehner LP, Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases: Am J Surg Pathol, 1995; 19(8); 859-72
5.. Shukla PS, Mittal K, Inflammatory myofibroblastic tumor in female genital tract: Arch Pathol Lab Med, 2019; 143(1); 122-29
6.. Cook JR, Dehner LP, Collins MH, Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study: Am J Surg Pathol, 2001; 25(11); 1364-71
7.. Haimes JD, Stewart CJR, Kudlow BA, Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1: Am J Surg Pathol, 2017; 41(6); 773-80
8.. Rabban JT, Zaloudek CJ, Shekitka KM, Tavassoli FA, Inflammatory myofibroblastic tumor of the uterus: A clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors: Am J Surg Pathol, 2005; 29(10); 1348-55
9.. Olgan S, Saatli B, Okyay RE, Koyuncuoglu M, Dogan E, Hysteroscopic excision of inflammatory myofibroblastic tumor of the uterus: A case report and brief review: Eur J Obstet Gynecol Reprod Biol, 2011; 157(2); 234-36
10.. Gupta N, Mittal S, Misra R, Inflammatory pseudotumor of uterus: An unusual pelvic mass: Eur J Obstet Gynecol Reprod Biol, 2011; 156(1); 118-19
11.. Gucer F, Altaner S, Mulayim N, Yapicier O, Invasive inflammatory pseudo-tumor of uterine cervix: A case report: Gynecol Oncol, 2005; 98(2); 325-28
12.. Abenoza P, Shek YH, Perrone T, Inflammatory pseudotumor of the cervix: Int J Gynecol Pathol, 1994; 13(1); 80-86
13.. Tan H, Wang B, Xiao H, Radiologic and clinicopathologic findings of inflammatory myofibroblastic tumor: J Comput Assist Tomogr, 2017; 41(1); 90-97
14.. Coffin CM, Patel A, Perkins S, ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor: Mod Pathol, 2001; 14(6); 569-76
15.. Li XQ, Hisaoka M, Shi DR, Zhu XZ, Hashimoto H, Expression of anaplastic lymphoma kinase in soft tissue tumors: An immunohistochemical and molecular study of 249 cases: Hum Pathol, 2004; 35(6); 711-21
16.. Lawrence B, Perez-Atayde A, Hibbard MK, TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors: Am J Pathol, 2000; 157(2); 377-84
17.. Gale N, Zidar N, Podboj J, Inflammatory myofibroblastic tumour of paranasal sinuses with fatal outcome: Reactive lesion or tumour?: J Clin Pathol, 2003; 56(9); 715-17
18.. Pickett JL, Chou A, Andrici JA, Inflammatory myofibroblastic tumors of the female genital tract are under-recognized: A low threshold for ALK immunohistochemistry is required: Am J Surg Pathol, 2017; 41(10); 1433-42
19.. Brivio E, Zwaan CM, ALK inhibition in two emblematic cases of pediatric inflammatory myofibroblastic tumor: Efficacy and side effects: Pediatr Blood Cancer, 2019; 66(5); e27645
20.. Kim SA, Lee SR, Huh J, IgG4-associated inflammatory pseudotumor of ureter: Clinicopathologic and immunohistochemical study of 3 cases: Hum Pathol, 2011; 42(8); 1178-84
21.. Li JY, Yong TY, Coleman M, Bilateral renal inflammatory pseudotumour effectively treated with corticosteroid: Clin Exp Nephrol, 2010; 14(2); 190-98
22.. Doski JJ, Priebe CJ, Driessnack M, Corticosteroids in the management of unresected plasma cell granuloma (inflammatory pseudotumor) of the lung: J Pediatr Surg, 1991; 26(9); 1064-66
23.. Applebaum H, Kieran MW, Cripe TP, The rationale for nonsteroidal anti-inflammatory drug therapy for inflammatory myofibroblastic tumors: A Children’s Oncology Group study: J Pediatr Surg, 2005; 40(6); 999-1003 ; discussion 1003
24.. Du X, Gao Y, Zhao H, Clinicopathological analysis of epithelioid inflammatory myofibroblastic sarcoma: Oncol Lett, 2018; 15(6); 9317-26
25.. Fordham AM, Xie J, Gifford AJ, CD30 and ALK combination therapy has high therapeutic potency in RANBP2-ALK-rearranged epithelioid inflammatory myofibroblastic sarcoma: Br J Cancer, 2020; 123(7); 1101-13
26.. Kubo N, Harada T, Anai S, Carboplatin plus paclitaxel in the successful treatment of advanced inflammatory myofibroblastic tumor: Intern Med, 2012; 51(17); 2399-401
27.. Tothova Z, Wagner AJ, Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors: Curr Opin Oncol, 2012; 24(4); 409-13
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








