09 December 2023: Articles 
Long-Term Indomethacin Treatment in a Chinese Child with Gitelman Syndrome: Case Report and Literature Review on its Efficacy and Tolerance
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease
Xiaoyan Peng1ABCEF, Chaoying Chen1AFG*, Juan Tu1DEF, Yuan Lin1BD, Huarong Li1BD, Haiyun Geng1BCDDOI: 10.12659/AJCR.941627
Am J Case Rep 2023; 24:e941627
Abstract
BACKGROUND: Gitelman syndrome (GS) is a rare inherited autosomal recessive salt-losing renal tubulopathy. Early-onset GS is difficult to differentiate from Bartter syndrome (BS). It has been reported in some cases that cyclooxygenase (COX) inhibitors, which pharmacologically reduce prostaglandin E2(PGE2) synthesis, are helpful for GS patients, especially in children, but the long-term therapeutic effect has not yet been revealed.
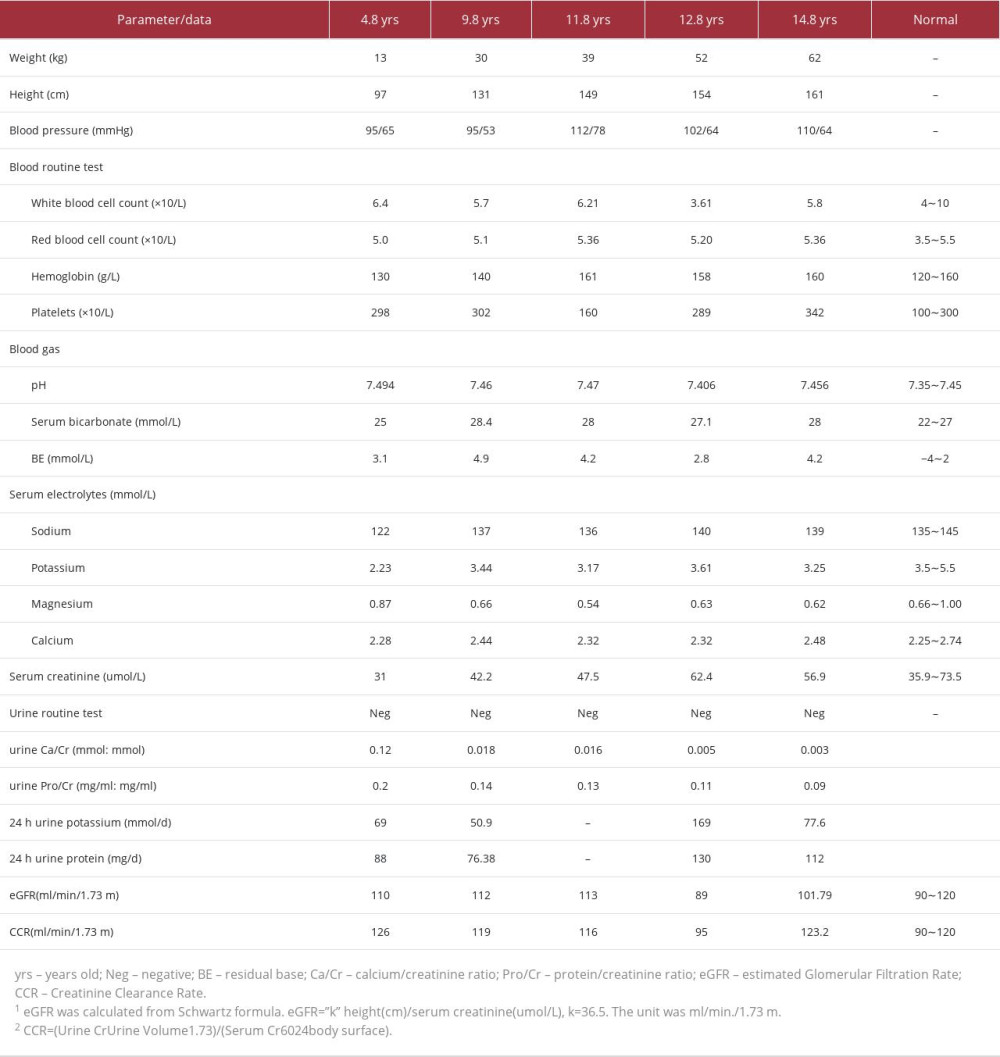
CASE REPORT: A 4-year-old boy was first brought to our hospital for the chief concern of short stature and growth retardation. Biochemical tests demonstrated severe hypokalemia, hyponatremia, and hypochloremic metabolic alkalosis. The patient’s serum magnesium was normal. He was diagnosed with BS and treated with potassium supplementation and indomethacin and achieved stable serum potassium levels and slow catch-up growth. At 11.8 years of age, the patient showed hypomagnesemia and a genetic test confirmed that he had GS with compound heterozygous mutations in the SLC12A3 gene. At the age of 14.8 years, when indomethacin had been taken for nearly 10 years, the boy reported having chronic stomachache, while his renal function remained normal. After proton pump inhibitor and acid inhibitor therapy, the patient’s symptoms were ameliorated, and he continued to take a low dose of indomethacin (37.5 mg/d divided tid) with good tolerance.
CONCLUSIONS: Early-onset GS in childhood can be initially misdiagnosed as BS, and gene detection can confirm the final diagnosis. COX inhibitors, such as indomethacin, might be tolerated by pediatric patients, and long-term therapy can improve the hypokalemia and growth retardation without significant adverse effects.
Keywords: Cyclooxygenase Inhibitors, Gitelman syndrome, Bartter Syndrome, Type 3
Background
Gitelman syndrome (GS, OMIM 263800) is an inherited salt-losing tubulopathy caused by loss-of-function mutations in the
We report a case of a Chinese boy who was first clinically diagnosed with BS and finally genetically diagnosed with GS. He was treated with long-term indomethacin and his growth retardation improved. Combined with a review of the relevant literature, this report may contribute to a deeper understanding of GS therapy.
Case Report
LITERATURE REVIEW:
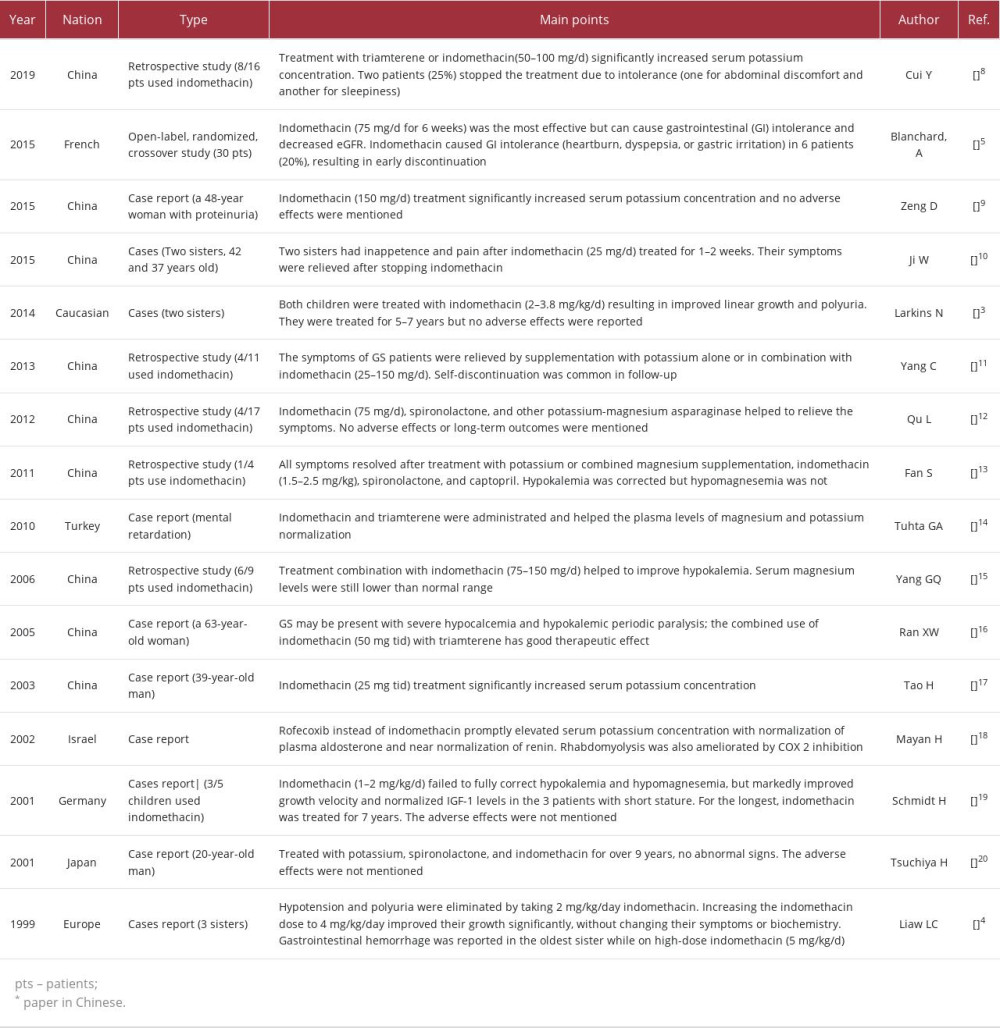
We entered the keywords “Gitelman syndrome” and “indo-methacin” in the PubMed, Wanfang, and SinoMed websites to search matched Chinese and English articles from 1999 to 2022. Following screening, 16 papers that reported GS patients treated with indomethacin were selected [3–5,8–20]. The main information and points are listed in Table 2. In most cases, the dose of indomethacin for adult patients was 25–150 mg/d [5,8–12,14–17,20], while it was 1–5 mg/kg/d for children [3,4,13,19]. There was a clear increase in the plasma potassium concentration, but the magnesium levels increased only slightly. Only 4 of the 16 papers reported adverse effects of indomethacin in GS patients [4,5,8,11]. Gastrointestinal (GI) intolerance (usually abdominal discomfort, heartburn, dyspepsia, or gastric irritation) was the most common problem, which was reported by 20–25% of the patients and resulted in early discontinuation. Blanchard et al reported that indomethacin (75 mg/d for 6 weeks) can cause decreased eGFR [5]. Larkins [3] and Schmidt [19] both reported children treated with indomethacin for more than 5 years, but did not report adverse effects.
Discussion
We report the case of a child with early-onset who was initially diagnosed with BS and later was confirmed to have GS by gene detection. With long-term indomethacin therapy for more than 10 years, his hypokalemia and growth retardation were both improved (Table 1). Indomethacin was well-tolerated, and there were no severe adverse effects. We also reviewed the literature to obtain a comprehensive understanding of indomethacin treatment in GS patients.
GS and BS patients have some overlapping clinical characteristics that make the diagnosis difficult, so genetic testing is the criterion standard for differentiating GS and BS. Because of the cost of genetic testing, the clinical diagnostic strategy for GS is always the preliminary choice in practice, and a careful history-taking, physical examination, and laboratory evaluation should be undertaken. Late-onset, hypomagnesemia, and hypocalciuria are features that differentiate GS from BS [21], but in recent years, with the development of sequencing technology, cases with normomagnesemia, normocalciuria, or early-onset GS have been reported [22,23]. The renal tubular function test was also proven to be effective in differentiation [24]. In our previous study [25], we evaluated the diagnostic utility of hypomagnesemia, hypocalciuria, and the hydrochlorothiazide (HCT) test in Chinese GS patients. We found that the sensitivity, specificity, and area under the curve (AUC) for hypomagnesemia and hypocalciuria were not sensitive enough to diagnose GS, but the HCT test was significantly superior (AUC 1.000, 95% CI 0.905–1.000). As we previously reported [22], normomagnesemic GS patients showed milder clinical manifestations, electrolyte abnormalities, metabolic alkalosis, and NCC dysfunction than the hypomagnesemic patients. It was also reported [26] that hypomagnesemia was not present at the early stage of GS, but presented years later. In this study, the child showed hypomagnesemia in the 7th year of disease and presented some challenges for the clinical diagnosis. We speculate that normomagnesemia might be a feature of the early-onset GS in children. Further study is needed to investigate the intensive mechanism involved in the association between the phenotype and genotype.
Growth retardation is an important comorbidity of renal tubular disease in children [1]. Clinical studies have demonstrated growth hormone deficiency in children with primary renal tubular disease, and laboratory data showed tissue-specific alterations in growth hormone and insulin-like growth factors-1 in hypokalemic animal models [27]. Maintaining a good electrolyte and acid-base balance can help promote catch-up growth. For this child, the only chief concern was growth retardation. Actively seeking medical attention helped him to receive an early diagnosis and intervention and finally improved his growth. The heights of his mother and father were 151 cm and 168 cm, respectively. At the age of 15, his height was 161 cm and almost reached his target height (166±8.5 cm). Both the child and his parents were satisfied with the treatment. Therefore, early diagnosis and treatment are important for improving the growth and prognosis of these children.
For treatment, when potassium and magnesium supplementation are not sufficient for persistent hypokalemia, potassium-sparing diuretics and renin angiotensin system blockers can be used. The most available and commonly used potassium-sparing diuretics was spironolactone, while its antiandrogenic effects were highly concerning in children and young adults, such as gynecomastia, hirsutism, erectile dysfunction, and menstrual irregularities [1]. In addition, spironolactone can aggravate the renal salt wasting and cause hypotension. In this case, spironolactone was taken at the early stage of disease but failed to improve the hypokalemia. The parents also worried about the adverse effects. For these reasons, when GS diagnose was made or when the GI complications appeared, they were preferred to maintain indomethacin instead of trying spironolactone again.
The application of COX inhibitors in GS and BS patients relied on the mechanism of elevated PGE2 levels. Existing evidence indicates that increased PGE2 might be secondary to salt loss rather than completely due to the primary disease. Even in BS, a traditional hyperprostaglandin E syndrome, not all patients show enhanced synthesis or excretion of PGE2 [28]. Our previous study [6] showed that the urinary PGE2 concentration varied in each individual and exhibited good correlation with serum chloride, serum magnesium, urinary calcium excretion, and arterial HCO3− concentration. GS patients with higher PGE2 showed higher percentages of nocturia and dyspnea, more severe metabolic alkalosis, a higher daily urinary excretion of potassium, and more severe NCC dysfunction. This provided reliable evidence for the application of COX inhibitors in GS patients. Larkins et al found increased urinary prostaglandin (PGE2) excretion in 2 children who showed a good response to indomethacin [3]. In this study, although we did not measure the level of PGE2, from the relieved clinical manifestations (severe hypokalemia and growth retardation), we supposed increased PGE2 levels in the child. For personalized therapy, we suggest that doctors measure PGE2 and its metabolite levels before treatment, and COX inhibitor may be a more appropriate choice for patients with elevated PGE2 levels.
The adverse effects of COX inhibitor can never be ignored. To the best of our knowledge, the most frequent adverse effect of COX inhibitors is gastrointestinal (GI) tolerability; mucosal injury in the upper GI tract is common with long-term use and affects up to 70% of long-term users [29]. According to the literature review, NSAIDs are rarely used and reported in GS patients. Only 4 studies reported the adverse effects of indomethacin, which were all regarding GI intolerance, at a proportion of 1/4 to 1/3. The longest treatment duration was reported by Tsuchiya [20], who treated a man with indomethacin for 9 years, but the dose and adverse effects were not mentioned in the paper. In our study, the GS patient was treated with indo-methacin for more than 10 years and showed good tolerance to the treatment, with only a slight stomachache. We can get more insight from reports of other diseases. Gasongo et al [30] analyzed the treatment of 19 children with BS from 1994 to 2016 (mean age at diagnosis was 0.9 months). In terms of adverse effects, a 2-year-old child developed necrotizing enterocolitis after 7 days of medication and then was treated again 6 months later and reported no adverse reactions. Two children with BS developed gastritis after 3 months of therapy. In the latest consensus and recommendations for BS [31], NSAIDs were recommended for use in early childhood, and gastric acid inhibitors were recommended for use together with NSAIDs.
However, they also mentioned that the risks of gastrointestinal and cardiovascular adverse effects need to be considered individually, especially if these medications are used in the first few weeks or months. The other important adverse effect of COX inhibitors is renal impairment. The medical literature reveals many adverse effects of NSAIDs on the kidneys, such as acute kidney injury (AKI), tubulointerstitial nephritis (TIN), nephrotic syndrome, and chronic kidney disease (CKD) [32]. Blanchard et al [5] reported that indomethacin significantly reduced eGFR by 10.0 ml/min/1.73 m2 after 6-week treatment. The influence of long-term NSAIDs treatment on renal function was reported in a cohort of children with juvenile idiopathic arthritis [33]. For those been treated by only NSAIDs, the cumulative proportion of patients free from kidney injury at 240 months from disease onset was 100%, indicating acceptable risk of long-term drug use. In this case, at age 14.8 years old, when indomethacin had been taken for nearly 10 years, the patient’s kidney function (Table 1) remained normal. His myocardial enzyme, electrocardiogram, and echocardiography were all normal, which reflected normal heart function. Because patients with risk factors of kidney impairment have far greater risk of adverse effects [34], it was recommended to create an individualized medication strategy for patients to control the nephrotoxicity. As mentioned before, we suggest a COX2 inhibitor as a more appropriate choice for patients with elevated PGE2 levels.
Conclusions
In conclusion, early-onset GS in childhood can be initially mis-diagnosed as BS, and gene detection can confirm the final diagnosis. COX inhibitors, such as indomethacin, might be tolerated by pediatric patients, and long-term therapy can improve hypokalemia and growth retardation without significant adverse effects.
References:
1.. Blanchard A, Bockenhauer D, Bolignano D, Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference: Kidney Int, 2017; 91(1); 24-33
2.. Seyberth HW, Schlingmann KP, Bartter- and Gitelman-like syndromes: Salt-losing tubulopathies with loop or DCT defects: Pediatr Nephrol, 2011; 26(10); 1789-802
3.. Larkins N, Wallis M, McGillivray B, Mammen C, A severe phenotype of Gitelman syndrome with increased prostaglandin excretion and favorable response to indomethacin: Clin Kidney J, 2014; 7(3); 306-10
4.. Liaw LC, Banerjee K, Coulthard MG, Dose related growth response to indo-metacin in Gitelman syndrome: Arch Dis Child, 1999; 81(6); 508-10
5.. Blanchard A, Vargas-Poussou R, Vallet M, Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome: J Am Soc Nephrol, 2015; 26(2); 468-75
6.. Peng X, Jiang L, Chen C, Increased urinary prostaglandin E2 metabolite: A potential therapeutic target of Gitelman syndrome: PLoS One, 2017; 12(7); e0180811
7.. Fletcher JT, Graf N, Scarman A, Nephrotoxicity with cyclooxygenase 2 inhibitor use in children: Pediatr Nephrol, 2006; 21(12); 1893-97
8.. Cui Y, Li M, Wang F, [Therapeutic efficacy of triamterene or indo-methacin in patients with Gitelman syndrome.]: Basic & Clinical Medicine, 2019; 11(39); 1603-6 [in Chinese]
9.. Zeng D, Li H, Gitelman syndrome concurrent with proteinuria: Chin J Diffic and Compl Cas, 2015(4); 416 [in Chinese]
10.. Ji W, Hei W, Yin Q, Zhang L, liao Z, [Diagnostic experience on familial Gitelman syndrome.]: Chinese J Endocrinol Metab, 2015; 31(12) [in Chinese]
11.. Yang C, Cao X, Fu J, Xiao H, Liao Z, Li Y, [Clinical analysis of Bartter syndrome and Gitelman syndrome.]: Chin J Clinicians(Electronic Edition), 2013(18); 8257-60 [in Chinese]
12.. Qu L, Zhang TT, Mu YM, [Clinical analysis of 17 cases of Gitelman syndrome.]: Nan Fang Yi Ke Da Xue Xue Bao, 2012; 32(3); 432-34 [in Chinese]
13.. Fan S, Zhang B, Wang W, [Clinical characteristics of primary renal tubular hypokalemic alkalosis.]: J Appl Clin Pediatr, 2011; 26(17) [in Chinese]
14.. Tuhta GA, Tuhta A, Erdogan M, Gitelman syndrome with mental retardation: A case report: J Nephrol, 2010; 23(5); 617-8
15.. Yang GQ, Zhao L, Xi WQ, [A clinical analysis of 9 cases of Gitelman syndrome.]: Zhonghua Nei Ke Za Zhi, 2006; 45(8); 650-53 [in Chinese]
16.. Ran XW, Wang C, Dai F, [A case of Gitelman’s syndrome presenting with severe hypocalcaemia and hypokalemic periodic paralysis.]: Sichuan Da Xue Xue Bao Yi Xue Ban, 2005; 36(4); 583-87 [in Chinese]
17.. Tao H, Dai W, Li Z, Jiang Y, [Gitelman syndrome(report of 2 cases).]: Chinese J Endocrinol Metab, 2003; 19(4) [in Chinese]
18.. Mayan H, Gurevitz O, Farfel Z, Successful treatment by cyclooxyenase-2 inhibitor of refractory hypokalemia in a patient with Gitelman’s syndrome: Clin Nephrol, 2002; 58(1); 73-76
19.. Schmidt H, Kabesch M, Schwarz HP, Kiess W, Clinical, biochemical and molecular genetic data in five children with Gitelman’s syndrome: Horm Metab Res, 2001; 33(6); 354-57
20.. Tsuchiya H, Kamoi K, Soda S, Gitelman’s syndrome first diagnosed as Bartter’s syndrome: Intern Med, 2001; 40(10); 1011-14
21.. Unwin RJ, Capasso G, Bartter’s and Gitelman’s syndromes: Their relationship to the actions of loop and thiazide diuretics: Curr Opin Pharmacol, 2006; 6(2); 208-13
22.. Jiang L, Peng X, Ma J, Normomagnesemic Gitelman syndrome patients exhibit a stronger reaction to thiazide than hypomagnesemic patients: Endocr Pract, 2015; 21(9); 1017-25
23.. Jiang L, Chen C, Yuan T, Clinical severity of Gitelman syndrome determined by serum magnesium: Am J Nephrol, 2014; 39(4); 357-66
24.. Peng X, Jiang L, Yuan T, Value of chloride clearance test in differential diagnosis of Gitelman syndrome: Zhongguo Yi Xue Ke Xue Yuan Xue Bao, 2016; 38(3); 275-82
25.. Peng X, Zhao B, Zhang L, Hydrochlorothiazide test as a tool in the diagnosis of gitelman syndrome in Chinese patients: Front Endocrinol (Lausanne), 2018; 9; 559
26.. Tammaro F, Bettinelli A, Cattarelli D, Early appearance of hypokalemia in Gitelman syndrome: Pediatr Nephrol, 2010; 25(10); 2179-82
27.. Gil-Peña H, Mejia N, Alvarez-Garcia O, Longitudinal growth in chronic hypokalemic disorders: Pediatr Nephrol, 2010; 25(4); 733-37
28.. Al Shibli A, Narchi H, Bartter and Gitelman syndromes: Spectrum of clinical manifestations caused by different mutations: World J Methodol, 2015; 5(2); 55-61
29.. Ho KY, Cardosa MS, Chaiamnuay S, Practice advisory on the appropriate use of NSAIDs in primary care: J Pain Res, 2020; 13; 1925-39
30.. Gasongo G, Greenbaum LA, Niel O, Effect of nonsteroidal anti-inflammatory drugs in children with Bartter syndrome: Pediatr Nephrol, 2019; 34(4); 679-84
31.. Konrad M, Nijenhuis T, Ariceta G, Diagnosis and management of Bartter syndrome: Executive summary of the consensus and recommendations from the European Rare Kidney Disease Reference Network Working Group for Tubular Disorders: Kidney international, 2021; 99(2); 324-35
32.. Drożdżal S, Lechowicz K, Szostak B, Kidney damage from nonsteroidal anti-inflammatory drugs – myth or truth? Review of selected literature: Pharmacol Res Perspect, 2021; 9(4); e00817
33.. Gicchino MF, Di Sessa A, Guarino S, Prevalence of and factors associated to chronic kidney disease and hypertension in a cohort of children with juvenile idiopathic arthritis: Eur J Pediatr, 2021; 180(2); 655-61
34.. Pham PC, Khaing K, Sievers TM, 2017 update on pain management in patients with chronic kidney disease: Clin Kidney J, 2017; 10(5); 688-97
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943070
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250