14 November 2023: Articles 
Immune Checkpoint Inhibitor-Induced Pure Red Cell Aplasia: A Review of 2 Cases in Metastatic Melanoma
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Diagnostic / therapeutic accidents, Rare disease, Adverse events of drug therapy, Clinical situation which can not be reproduced for ethical reasons
Libardo Rueda PradaDOI: 10.12659/AJCR.941789
Am J Case Rep 2023; 24:e941789
Abstract
BACKGROUND: Immunotherapy is a novel treatment offering an alternative to traditional chemotherapeutic agents for different malignancies. Hematologic adverse reactions (HARs) related to immune checkpoint inhibitors (ICIs) are uncommon. Pure red cell aplasia (PRCA) is a rare hematologic complication of ICI therapy in metastatic melanoma with significant mortality risk despite treatment with steroids or immunosuppressive therapy. For unexplained acute anemia after exclusion of other causes, performing bone marrow biopsy is imperative to diagnose PRCA and rule out involvement of bone marrow by primary tumor. HARs can occur during ICI therapy or even after ICI therapy is stopped. ICI rechallenge, even after the development of HARs, is considered in some patients with good response to treatment of HARs from ICIs. Recurrence of HARs with the same or different type of reaction is seen in some patients.
CASE REPORT: Two cases of ICI-induced PRCA were confirmed on bone marrow biopsy after dual ICI treatment with nivolumab and ipilimumab in metastatic melanoma. In case 2, PRCA was successfully treated with steroids and later rechallenged with single-agent nivolumab, causing mild ICI-induced immune thrombocytopenia, which did not require treatment with steroids.
CONCLUSIONS: It is crucial to increase clinician awareness of the possibility of PRCA development not only during treatment with ICI but also after finishing treatment with ICI; there is high mortality associated with missing an opportunity to diagnose and treat PRCA on time with favorable results. ICI rechallenge can be considered in patients who showed response to immunotherapy, especially those with limited alternative therapeutic options.
Keywords: Immune Checkpoint Inhibitors, Immunotherapy, Melanoma, Red-Cell Aplasia, Pure
Background
Pure red cell aplasia (PRCA) is a rare syndrome manifested by marked reticulocytopenia, relative absence of erythroid precursors on bone marrow examination, and normocytic normochromic anemia due to erythropoiesis failure [1–3]. Congenital forms of PRCA are Diamond-Blackfan anemia and Pearson syndrome, but these forms are much less common than acquired PRCA, which is further classified as primary or secondary [1–3]. Secondary acquired PRCA is the most common form, and its etiologies vary, with infections, autoimmune conditions, solid tumors, and medication-related adverse effects being the most common. Multiple medications have been previously reported to cause PRCA, and, for most of these, the exact mechanism causing PRCA is unclear. Commonly used medications associated with secondary PRCA include azathioprine, allopurinol, carbamazepine, clopidogrel, linezolid, tacrolimus, valproic acid, and trimethoprim-sulfamethoxazole [4–6]. Immune checkpoint inhibitors (ICIs) are promising immunotherapy agents with expanding indications for multiple cancers. Their association with PRCA is established but remains rare [7,8]. Here we report 2 cases of PRCA related to immunotherapy for metastatic melanoma and discuss differential diagnosis and management.
Case Reports
CASE 1:
A 56-year-old woman presented to the Oncology Clinic for evaluation of acute anemia. Her past medical history included stage IV BRAF wild-type metastatic cutaneous melanoma to the brain, lung, liver, and bones. She previously received pembrolizumab, experiencing disease progression, and recently completed the second cycle of dual immunotherapy with nivolumab and ipilimumab 4 weeks before presentation. She had no history of prior gastrointestinal bleeding.
Her initial vital signs were within normal limits. Physical examination was significant for a pale, alert patient. The remainder of her physical examination was within normal limits, except for a 3-cm sacral wound with mild surrounding redness, greenish discharge, and no bone exposure. Initial laboratory work-up was remarkable for normocytic anemia, with a hemoglobin level of 7.4 g/dL (baseline 13.1 g/dL, 3 weeks prior), chronic hyponatremia, with a sodium level stable at 133 mmol/L, creatinine level of 0.97 mg/dL (at baseline), albumin level of 3.3 g/dL, and mild chronic aspartate transaminase and alkaline phosphatase elevation at 61 U/L and 181 U/L, respectively.
The urinalysis results were suggestive of infection. Additional laboratory workup is summarized in Table 1. No leukopenia or thrombocytopenia was observed. There was persistent reticulocytopenia, which improved approximately 1 month after the ICI was stopped. Despite an elevated D-dimer level, disseminated intravascular coagulation was considered less likely given a fibrinogen level >100 mg/dL, absence of thrombocytopenia, minimally elevated prothrombin time/international normalized ratio, normal activated partial prothrombin time, and absence of clinical signs of bleeding or thrombosis. Hemolysis seemed less likely given the chronic lactate dehydrogenase elevation in the setting of liver metastasis, absence of hyperbilirubinemia, negative Coombs test, and absence of schistocytes on peripheral blood smear. The low haptoglobin level could have been related to metastatic liver disease.
Intravenous (i.v.) ceftriaxone, vancomycin, and flagyl were initiated to cover for a possible infected sacral wound and urinary tract infection. Her hemoglobin level dropped in the first 24 h, to 5.8 g/dL. Her hemoglobin level improved after a total of 3 units of packed red blood cells. Her reticulocyte count was persistently low. The urine culture result was positive for pan-sensitive E. coli. Her sacral ulcer improved with wound care measures. There were no signs of abscess. Antibiotics were deescalated to i.v. ceftriaxone, and 7 days of treatment were completed. The Hematology Department was consulted for suspected PRCA related to immunotherapy. Bone marrow biopsy results showed slight hypercellularity (60%) with markedly decreased erythroid precursors (<5% of total) with minimal erythroid maturation, consistent with PRCA, while morphology and immunohistochemistry showed no evidence of involvement of metastatic melanoma (Figure 1). However, additional bone marrow studies (chromosome karyotype and Next-GeneSequencing) were both abnormal yet inconclusive as to the etiology from myelodysplastic syndrome/clonal cytopenia of undetermined significance and/or melanoma. PRCA diagnosis was considered based on the bone marrow findings mentioned, and no other conclusive alternative etiology was found on further testing. An immunotherapy rechallenge was not considered, given the rapid disease progression, poor performance status, and concern of anemia relapse. Her hemoglobin level remained stable, with no new transfusions required. She was not a candidate for further systemic therapy or clinical trial, given her decreased performance status. The patient transitioned her care to hospice.
CASE 2:
A 25-year-old woman presented to the Oncology Clinic for evaluation of fever and fatigue. She was diagnosed with BRAF wild-type metastatic mucosal melanoma in the anus 3 months earlier and had an excision of the anal mass 10 days after initial diagnosis. Since then, she had received frontline dual immuno-therapy with nivolumab (1 mg/kg) and ipilimumab (3 mg/kg). She had completed 3 cycles of immunotherapy, with the most recent one 5 days before presentation. Two days after her last treatment, she developed fatigue and a right-sided facial droop and was evaluated in the Emergency Department. At that time, her hemoglobin level was 6.9 g/dL (baseline 9.3 g/dL), and she received 2 units of packed red blood cells. The computed tomography (CT) scan of the head and magnetic resonance imaging (MRI) of the brain were within normal limits. Possible Bell’s palsy was diagnosed.
During the clinic visit, her vital signs were remarkable for a temperature of 39.3 °C. The rest of her vital signs were within normal limits. At the physical examination, she was in no acute distress. The rest of her physical examination was within normal limits, except for an erythematous macular rash on both cheeks, sparing the nasolabial folds, and neurologic examination with no signs of focalization, except for right-sided peripheral VII cranial nerve palsy.
Initial laboratory workup was significant for normocytic anemia, with a hemoglobin level of 7.1 g/dL, unremarkable chemistry panel, with creatinine level of 0.85 mg/dL, and normal liver enzyme and alkaline phosphatase levels. The remaining laboratory results are summarized in Table 2. CT of the chest/ abdomen/pelvis with i.v. contrast showed progression of the metastatic lymphadenopathy in the pelvis and progressive enlargement of a metastatic lesion in the right ilium. Blood culture results were negative. Antibiotics were not initiated, owing to a low suspicion for infection. Disseminated intravascular coagulation was considered less likely. Hemolysis was considered in the differential diagnosis, based on additional test results (see Table 2).
The patient was treated with i.v. Solu-Medrol 1 g daily for 5 days for probable immunotherapy-induced PRCA, with a component of possible autoimmune hemolytic anemia. Her hemoglobin level dropped in the first day to 5.4 g/dL. She received a transfusion with 2 units of packed red blood cells. Her hemoglobin level improved to 6.9 g/dL on day 2 and to 9.5 g/dL on day 3, without additional transfusion needed. A bone marrow aspirate and biopsy (Figure 2) showed a slightly hypercellular marrow for age (90%), with decreased and markedly left-shifted erythropoiesis and increased granulopoiesis and megakaryocytes. There was marked anisopoikilocytosis with occasional microcytic cells and slight elliptocytes, white blood cells without cytologic abnormalities, and platelets without cytologic abnormalities. The myeloid: erythroid ratio was >10: 1. Erythroid precursors quantity was decreased, left shifted maturation with predominantly pronormoblasts. Myeloid precursors quantity was increased, normal maturation. Iron staining was done, showing storage iron was normal, sideroblasts were absent, and ring sideroblasts were not seen. There was no involvement by metastatic melanoma. Parvovirus B19 staining was negative. Solu-Medrol i.v. was followed by 5 days of i.v. immune globulin (IVIG), cyclosporine 100 mg twice daily, and prednisone. Ipilimumab and nivolumab were discontinued.
Four months later, while being off treatment, she was noticed to have new multifocal lytic bone lesions and a new lung nodule, so she was re-started on single-agent nivolumab. After 2 cycles, she developed acute thrombocytopenia (platelet count of 66×109/L, which resolved after a course of prednisone), suggestive of mild immune thrombocytopenia secondary to ICI; however, her hemoglobin level remained stable, and therefore treatment was continued. Four months after having been on nivolumab, MRI of the femur suggested new metastatic disease, and nivolumab was stopped. Carboplatin, paclitaxel, and pembrolizumab were then initiated as salvage therapy. She had a partial response after 3 cycles of salvage chemo-immunotherapy. Unfortunately, she experienced thrombocytopenia and therefore continued treatment with carboplatin, reduced to AUC 4. After 6 cycles, the patient had a positive response with no new metastatic disease and was placed on a treatment holiday per her preference. Chemo-immunotherapy was reinitiated 3 months later because of symptomatic disease progression. She was able to tolerate the treatment for the next 6 months, until she was found to have cerebral metastatic disease, for which she received radiation therapy, followed by admissions for rectal bleeding and lumbar spine pathologic fracture, causing cauda equina. She died 6 months later.
Discussion
Our immune system plays an important role in the battle against cancer. Immune checkpoints are crucial self-tolerance pathways that prevent the immune system from attacking healthy cells. Cancer cells downregulate the immune system by multiple mechanisms, including production of immunosuppressive cytokines that stimulate inhibitory immune checkpoints [9]. Immunotherapy is a revolutionary therapy that enhances a patient’s immune system to destroy tumor cells. With the introduction of ipilimumab in 2011, followed by pembrolizumab in 2014, ICIs have transformed cancer management, offering a usually more tolerable alternative to the systemic cytotoxic therapy. The use of ICIs has shown benefits in the treatment of different types of malignancies, including melanoma, Hodgkin’s lymphoma, and lung, bladder, and renal cancer [10].
Unfortunately, immunotherapy can cause immune-related adverse events (irAEs) and compromise almost any organ or system. The skin, digestive, pulmonary, renal, and endocrine systems are the most affected [11]. Hematologic adverse reactions (HARs) to immunotherapy are not common and include thrombocytopenia, pancytopenia or immune aplastic anemia, neutropenia, hemolytic anemia, cytokine release syndrome with hemophagocytic syndrome, bicytopenia, and PRCA [12]. Immune-mediated thrombocytopenia and hemolytic anemia are among the most common HARs seen, usually involving the use of the ICIs nivolumab and pembrolizumab [13]. Ipilimumab has shown the shortest time from therapy initiation to onset of HARs [13]. It is important to keep monitoring blood cell counts even after discontinuation of treatment, given that HAR recurrence has been seen in approximately 8.9% of cases [13]. The mortality from HARs related to ICIs is variable and dependent on the type of disturbance, with the highest seen being aplastic anemia at 54.5%, and average mortality for all adverse reactions between 12% and 15.5% [13,14]. Hence, it is important to increase clinician awareness of this condition to provide prompt treatment and avoid significant mortality.
There are reports of nivolumab-induced autoimmune hemolytic anemia [15,16], some which are associated with PRCA [17]. Rare cases of PRCA have been reported without hemolysis, such as the case reported by Yuki et al of a patient with cardiac metastatic melanoma who developed PRCA at 21 months of being on nivolumab [18]. There are several case reports of aplastic anemia in patients with metastatic melanoma and other malignancies treated with dual immunotherapy [13,19–21]. However, cases of PRCA with dual immunotherapy in patients with metastatic melanoma have been rarely reported. A case series review of 15 cases of new-onset PRCA related to immunotherapy from the Food and Drug Administration’s Adverse Event Reporting System from 2011 to 2022 reported 9 cases related to metastatic melanoma, among which 6 were related to single immunotherapy either with pembrolizumab, ipilimumab or nivolumab, and only 3 were related to dual immunotherapy with ipilimumab and nivolumab [7].
PRCA is rare and usually presents as a normocytic, normochromic anemia with a persistent low reticulocyte count and significant decreased or absent red blood cell precursors in bone marrow (< 1% erythroblasts or few proerythroblasts and/or basophilic erythroblasts not exceeding 5% of the marrow differential count). Iron stains are usually normal. PRCA can be congenital or acquired. Acquired PRCA can be caused by autoimmune diseases, neoplasms invading bone marrow, medications, such as azathioprine and procainamide, or can be related to infections, such as parvovirus B19.
Our patients did not have a history of a pre-existing autoimmune disease, which if present and active may have increased their risk of developing severe irAEs with the use of ICIs. A multicenter study of a small cohort of patients with meta-static melanoma treated with ipilimumab who had a pre-existing autoimmune diseases showed a higher incidence of irAEs and autoimmune disorder flare-up [22]. These concerns led to exclude patients with autoimmune diseases from clinical trials. Further studies were done to clarify the risk-benefit ratio of ICIs in patients with cancer and pre-existing auto-immune diseases. These studies indicated that the use of ICIs may be safe in this patient population and is not an absolute indication to discontinue ICI therapy. However, there may be still a risk of severe and fatal disease [23,24]. A more recent study showed similar efficacy and safety with ICI use in patients with cancer without and with pre-existing autoimmune disease. However, impaired T-cell activation in patients with active pre-existing autoimmune disease predisposes them to worse outcomes during ICI therapy. On the contrary, the development of immune irAEs in patients with autoimmune disease, which was found to be usually mild, may be a sign of T-cell activation, which is associated with superior immuno-therapy efficacy and better outcomes [25].
Neurological irAEs are seen in <1% of patients on ICIs [26]. Bell’s palsy, as seen in case 2, has been rarely reported in patients on immunotherapy [26–28]. A single-center retrospective study of 353 patients treated with immunotherapy between 2015 and 2018 found 5 cases of Bell’s palsy, from which 3 cases were related to dual immunotherapy in patients with metastatic melanoma [27]. In some cases, it is difficult to differentiate a paraneoplastic syndrome manifestation related to underlying malignancy from neurological irAEs secondary to ICIs.
Anemia observed in oncologic patients can be multifactorial. Hence, bone marrow biopsy is important to confirm the diagnosis of PRCA and is critical to rule out direct tumor involvement of the bone marrow to determine the potential cause of PRCA and to guide further treatment. In case 1, infection was unlikely to be the major cause for the profound anemia, since the laboratory findings were not compatible with infectious hemolytic anemia and there were no signs of severe systemic infection. In addition, our patients were not on other medications that could have explained their anemia, and other etiologies, such as disseminated intravascular coagulation, and infections, such as parvovirus B19 infection, were ruled out. Hemolysis was considered unlikely in case 1 but likely in case 2, based on hemolysis workup. Although haptoglobin is commonly used as one of the markers for hemolysis, its value should be interpreted in conjunction with other hemato-logic evaluations to establish the diagnosis of hemolysis. In addition, an abnormal haptoglobin level can be seen in other conditions, such as metastatic carcinomas or cirrhosis, in the absence of hemolysis [29]. Low haptoglobin in case 1 was considered possibly related to liver dysfunction from metastatic disease. The patient in case 1 had chronic elevated lactate dehydrogenase possible secondary to metastatic disease and had no acute change during this hospital admission
The patient in case 1 did not require any additional intervention more than supportive blood transfusions. But in other cases, such as case 2, treatment with high-dose steroid, IVIG, and chronic immunosuppressant is required, and it is important to consider rechallenge with immunotherapy after clinical recovery. Final management decisions are individualized and made based on initial workup, severity, and clinical course, despite the initial similar diagnosis of PRCA. Other types of HARs could be seen with ICI rechallenge, such as the one seen in case 2
The exact mechanism of PRCA related to immunotherapy is unknown, but like other irAEs, it is presumed to be related to widespread activation of cytotoxic T cells, production of anti-red blood cell (or erythroid precursors) antibodies, and direct damage to erythroblasts [30]. In fact, activated CD8+ lymphocytes seen in bone marrow specimens of patients with PRCA support this theory [31]. Other studies have suggested a possible production of red blood cell antibodies against erythroid precursors or erythropoietin, especially with ipilimumab [16,32]. Because of the rarity of PRCA related to immunotherapy, it is unclear whether PRCA is more likely to occur in certain patient populations, such as according to sex, age, and other comorbidities. In addition, the immunotherapy regimens used that are associated with PRCA also need to be evaluated in further studies, especially given the introduction of a newer generation of ICIs over the past few years.
Treatment of PRCA related to immunotherapy includes corticosteroids, supportive management with transfusions as needed, and the addition of cyclosporine in refractory cases. Other HARs may require additional treatments, such as IVIG, rituximab, or infliximab, depending on the type of HAR shown. ICI rechallenge is considered when indicated after a patient has recovered. This represents a risk for HAR recurrence, which has been seen in between 33% and 43% of cases [13,33]. However, not all cases have shown recurrence of HAR with the rechallenge of ICI therapy [13]. In addition, patients should be closely monitored, as they are at risk of developing another type of HAR, as was seen in case 2, in which the patient did not have recurrent PRCA but did develop acute thrombocytopenia with the immunotherapy rechallenge. Additional studies are needed to identify the risk factors that can predispose patients to develop HAR recurrence after an ICI rechallenge. It is not well known if this recurrence can be related to the long half-life of these antibodies or prolonged receptor T-cell occupancy. Most cases of ICI-induced PRCA had fatal outcomes, despite steroid or immunosuppressive treatment [7], unlike case 2.
Conclusions
Immunotherapy is a novel and revolutionary treatment being widely used for different types of malignancies. There is a significant risk of irAEs with the use of immunotherapy. HARs to immunotherapy are not common and carry a significant mortality risk. PRCA secondary to immunotherapy in metastatic melanoma is rare and carries significant mortality risk, despite treatment with steroids or immunosuppressive therapy. Bone marrow biopsy is crucial for diagnosis and for performing additional studies to rule out other etiologies, such as direct tumor invasion to the bone marrow. Patients with PRCA related to immunotherapy are at risk of developing other types of HARs. It is important to increase clinician awareness of this risk in order to provide prompt initial treatment and to continue monitoring patients for any other HARs during immunotherapy rechallenge, particularly in patients who are candidates and have no other options for treatment.
Figures
References:
1.. Means RT, Pure red cell aplasia: Blood, 2016; 128(21); 2504-9
2.. Gurnari C, Maciejewski JP, How I manage acquired pure red cell aplasia in adults: Blood, 2021; 137(15); 2001-9
3.. Sawada K, Hirokawa M, Fujishima N, Diagnosis and management of acquired pure red cell aplasia: Hematol Oncol Clin North Am, 2009; 23(2); 249-59
4.. Chao SC, Yang CC, Lee JY, Hypersensitivity syndrome and pure red cell aplasia following allopurinol therapy in a patient with chronic kidney disease: Ann Pharmacother, 2005; 39(9); 1552-56
5.. Koduri PR, Vanajakshi S, Anuradha R, Azathioprine-associated pure red cell aplasia in renal transplant recipients: A report of two cases: Ann Hematol, 2014; 93(2); 329-30
6.. Yang XY, Chen L, Gu JN, Linezolid-induced pure red cell aplasia: A case report: Infect Drug Resist, 2022; 15; 3847-56
7.. Guo Q, Gao J, Guo H, Immune checkpoint inhibitor-induced pure red cell aplasia: Case series and large-scale pharmacovigilance analysis: Int Immunopharmacol, 2023; 114; 109490
8.. Saliba AN, Xie Z, Higgins AS, Immune-related hematologic adverse events in the context of immune checkpoint inhibitor therapy: Am J Hematol, 2021; 96(10); E362-E67
9.. Vinay DS, Ryan EP, Pawelec G, Immune evasion in cancer: Mechanistic basis and therapeutic strategies: Semin Cancer Biol, 2015; 35(Suppl.); S185-S98
10.. Marin-Acevedo JA, Kimbrough EO, Lou Y, Next generation of immune checkpoint inhibitors and beyond: J Hematol Oncol, 2021; 14(1); 45
11.. Brahmer JR, Lacchetti C, Schneider BJ, Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline: J Clin Oncol, 2018; 36(17); 1714-68
12.. Michot JM, Lazarovici J, Tieu A, Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage?: Eur J Cancer, 2019; 122; 72-90
13.. Ghanem P, Marrone K, Shanbhag S, Current challenges of hematologic complications due to immune checkpoint blockade: A comprehensive review: Ann Hematol, 2022; 101(1); 1-10
14.. Davis EJ, Salem JE, Young A, Hematologic complications of immune checkpoint inhibitors: Oncologist, 2019; 24(5); 584-88
15.. Palla AR, Kennedy D, Mosharraf H, Doll D, Autoimmune hemolytic anemia as a complication of nivolumab therapy: Case Rep Oncol, 2016; 9(3); 691-97
16.. Schwab KS, Heine A, Weimann T, Development of hemolytic anemia in a nivolumab-treated patient with refractory metastatic squamous cell skin cancer and chronic lymphatic leukemia: Case Rep Oncol, 2016; 9(2); 373-78
17.. Nair R, Gheith S, Nair SG, Immunotherapy-associated hemolytic anemia with pure red-cell aplasia: N Engl J Med, 2016; 374(11); 1096-97
18.. Yuki A, Takenouchi T, Takatsuka S, Ishiguro T, A case of pure red cell aplasia during nivolumab therapy for cardiac metastatic melanoma: Melanoma Res, 2017; 27(6); 635-37
19.. Helgadottir H, Kis L, Ljungman P, Lethal aplastic anemia caused by dual immune checkpoint blockade in metastatic melanoma: Ann Oncol, 2017; 28(7); 1672-73
20.. Meyers DE, Hill WF, Suo A, Aplastic anemia secondary to nivolumab and ipilimumab in a patient with metastatic melanoma: A case report: Exp Hematol Oncol, 2018; 7; 6
21.. Younan RG, Raad RA, Sawan BY, Said R, Aplastic anemia secondary to dual cancer immunotherapies a physician nightmare: Case report and literature review: Allergy Asthma Clin Immunol, 2021; 17(1); 112
22.. Johnson DB, Sullivan RJ, Ott PA, Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders: JAMA Oncol, 2016; 2(2); 234-40
23.. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME, Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review: Ann Intern Med, 2018; 168(2); 121-30
24.. Haanen J, Ernstoff MS, Wang Y, Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy: Ann Oncol, 2020; 31(6); 724-44
25.. Han CY, Fitzgerald C, Lee M, Association between toxic effects and survival in patients with cancer and autoimmune disease treated with checkpoint inhibitor immunotherapy: JAMA Oncol, 2022; 8(9); 1352-54
26.. Kichloo A, Albosta MS, Jamal SM, Atezolizumab-induced Bell’s palsy in a patient with small cell lung cancer: J Investig Med High Impact Case Rep, 2020; 8; 2324709620965010
27.. Yuen C, Reid P, Zhang Z, Facial palsy induced by checkpoint blockade: A single center retrospective study: J Immunother, 2019; 42(3); 94-96
28.. Takemura K, Yamanaka T, Hayashida M, Bell’s palsy during rechallenge of immune checkpoint inhibitor: IJU Case Rep, 2023; 6(2); 144-46
29.. Shih AW, McFarlane A, Verhovsek M, Haptoglobin testing in hemolysis: Measurement and interpretation: Am J Hematol, 2014; 89(4); 443-47
30.. Young NS, Abkowitz JL, Luzzatto L, New insights into the pathophysiology of acquired cytopenias: Hematology Am Soc Hematol Educ Program, 2000; 18-38
31.. Zhuang J, Du J, Guo X, Clinical diagnosis and treatment recommendations for immune checkpoint inhibitor-related hematological adverse events: Thorac Cancer, 2020; 11(3); 799-804
32.. Simeone E, Grimaldi AM, Esposito A, Serious haematological toxicity during and after ipilimumab treatment: A case series: J Med Case Rep, 2014; 8; 240
33.. Delanoy N, Michot JM, Comont T, Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: A descriptive observational study: Lancet Haematol, 2019; 6(1); e48-e57
Figures
Tables
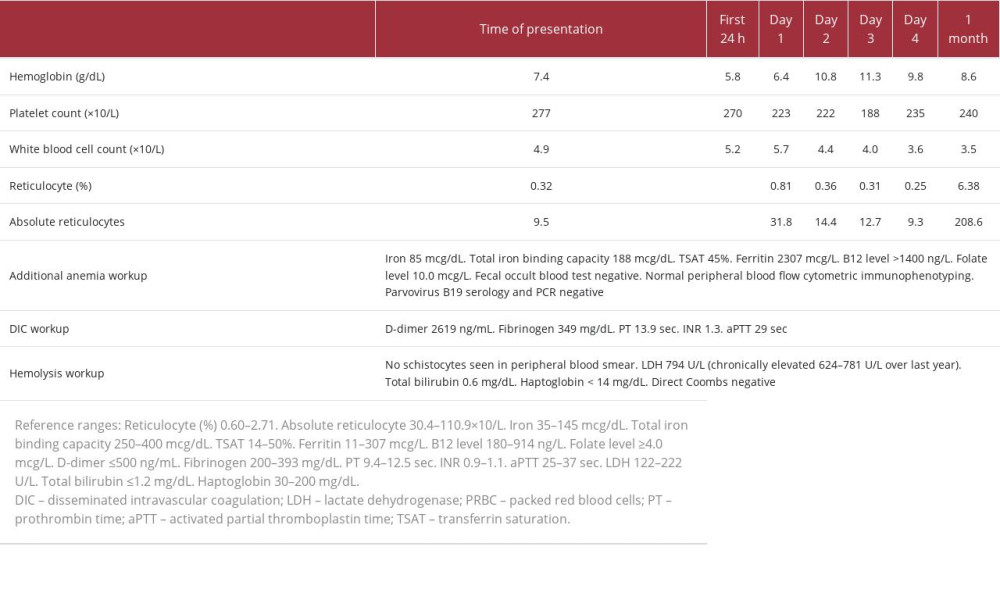 Table 1.. Case 1. Summary of laboratory workup and timeline of hospital interventions.
Table 1.. Case 1. Summary of laboratory workup and timeline of hospital interventions.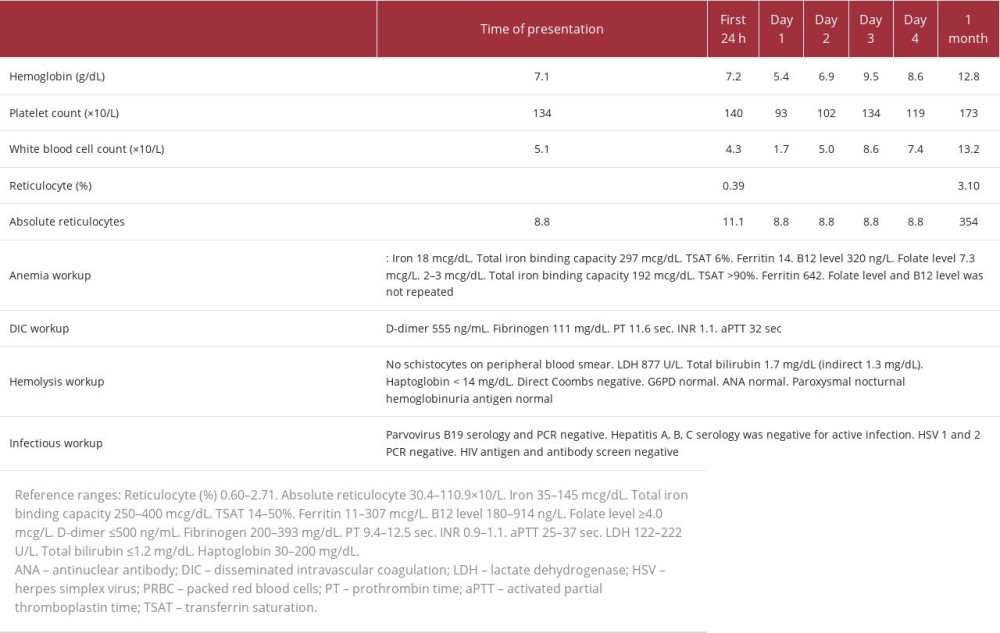 Table 2.. Case 2. Summary of laboratory workup and timeline of hospital interventions.
Table 2.. Case 2. Summary of laboratory workup and timeline of hospital interventions. Table 1.. Case 1. Summary of laboratory workup and timeline of hospital interventions.
Table 1.. Case 1. Summary of laboratory workup and timeline of hospital interventions. Table 2.. Case 2. Summary of laboratory workup and timeline of hospital interventions.
Table 2.. Case 2. Summary of laboratory workup and timeline of hospital interventions. In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250








