25 November 2023: Articles 
Primary Thymic Hodgkin Lymphoma Coexisting with Thymoma and Myasthenia Gravis: A Case Report
Challenging differential diagnosis, Unusual or unexpected effect of treatment, Rare disease, Rare coexistence of disease or pathology
Sarah Almuqbil1BEF*, Amal AlHarbi1BEF, Fatimah S. Alzouri1BEF, Hatem Yazeed Elbawab2ABEF, Noor S. Alsafwani3BE, Zahra Alkhunaizy3BEDOI: 10.12659/AJCR.941792
Am J Case Rep 2023; 24:e941792
Abstract
BACKGROUND: Myasthenia gravis is a neuromuscular disorder that is strongly associated with thymoma. Although the presence of myasthenia gravis with other tumors is uncommon, approximately 50% of patients with thymoma have myasthenia gravis. Thymic Hodgkin lymphoma should be considered due to the multiple reported cases of patients with myasthenia gravis and Hodgkin lymphoma. In this report, we present the case of 24-year-old woman with myasthenia gravis who was incidentally found to have coexisting thymoma with thymic Hodgkin lymphoma.
CASE REPORT: A 24-year-old woman with a known case of vitiligo presented with a 2-year history of diplopia and incidental anterior mediastinal mass. Following investigations, myasthenia gravis was diagnosed and managed by pyridostigmine, prednisolone, and azathioprine. Regarding the anterior mediastinal mass, thymoma was suspected based on the presence of myasthenia gravis and radiological findings. She underwent extended transsternal thymectomy. The final histopathological report of the dissected thymus disclosed Hodgkin lymphoma pathology coexisting with thymoma. After the diagnosis of Hodgkin lymphoma nodular sclerosis type IIA was confirmed, 6 cycles of chemotherapy were administered. Four years of follow-up revealed no evidence of Hodgkin lymphoma. However, her symptoms of myasthenia gravis persisted despite Hodgkin lymphoma remission.
CONCLUSIONS: There is an unclear association between myasthenia gravies and Hodgkin lymphoma. Prior reports revealed regression of myasthenia gravies following Hodgkin lymphoma management, which suggests that myasthenia could be a complication of Hodgkin lymphoma. However, in our case, myasthenia gravis persisted after Hodgkin lymphoma management; therefore, further studies are needed to explore this association.
Keywords: Hodgkin Disease, Myasthenia Gravis, Thymoma, Mediastinal Neoplasms, Paraneoplastic Syndromes
Background
Thymoma is an anterior mediastinal tumor derived from the epithelial cell of the thymus. It is the most common mass of the anterior mediastinum [1]. Thymoma has a strong relationship with paraneoplastic syndromes, particularly myasthenia gravis, which is an autoimmune disorder that affects acetylcholine receptors at the neuromuscular junction in the skeletal muscle [1]. Patients with myasthenia gravis tend to present with ocular, bulbar, and limb muscles weakness, and approximately 50% of patients with thymoma experience symptoms suggestive of myasthenia gravis [1].
Hodgkin lymphoma is a monoclonal lymphoid tumor that commonly affects mediastinal lymph nodes [2,3]. Primary thymic Hodgkin lymphoma affection without lymphadenopathy is rare [3]. While myasthenia gravis is frequently associated with thymoma, its relationship with other anterior mediastinal tumors, particularly primary thymic Hodgkin lymphoma, is rarely reported [4].
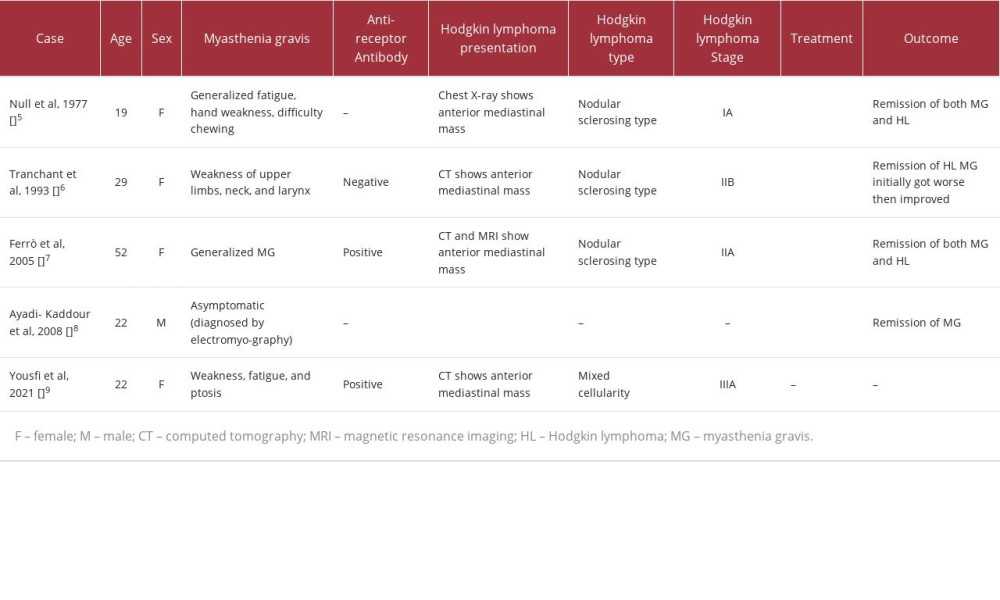
To the best of our knowledge, only 5 cases reported the association between myasthenia gravis and thymic Hodgkin lymphoma, as summarized in Table 1. Null et al documented a case of a young woman with myasthenia gravis who had a history of fatigue, hand weakness, and difficulty chewing [5]. In that case, myasthenia gravis was associated with an anterior mediastinal mass on chest radiography, and initially thymoma was suspected [5]. After thymectomy, the diagnosis of isolated primary thymic Hodgkin lymphoma of nodular sclerosing type, stage IA was confirmed. Surgical resection of the thymic lesion was followed by complete remission of myasthenia gravis symptoms. The patient underwent 2 courses of radiotherapy as well [5]. Similarly, Tranchant et al reported a young woman who presented with upper limb, neck, and larynx weakness and was diagnosed with seronegative myasthenia gravis [6]. In that case, a computed tomography (CT) scan showed an anterior mediastinal mass, and the lesion was resected. The pathology report revealed thymic Hodgkin lymphoma of nodular sclerosing type, stage IIB. After the surgical resection of thymus, she was given 3 cycles of chemotherapy, and radiotherapy was provided later. Initially after Hodgkin lymphoma treatment, she experienced worsening of myasthenia gravis symptoms, but then the symptoms started to improve slowly, and the corticosteroid was stopped [6]. Additionally, Ferrò et al reported a middle-aged female patient diagnosed with seropositive generalized myasthenia gravis [7]. After starting medical treatment of myasthenia gravis, radiological imaging suggested thymoma. The patient underwent thymectomy, and the tissues sent for cytopathology analysis showed thymic Hodgkin lymphoma of nodular sclerosing type, stage IIA [7]. The symptoms of myasthenia gravis resolved, with a significant decrease of anti-nicotinic acetylcholine receptors following the surgical resection, chemotherapy, and radiotherapy [7]. Moreover, Ayadi-Kaddour et al reported a young male patient who presented with thoracic symptoms [8]. Chest radiography and CT showed an anterior mediastinal mass. Since thymoma was suggested, electromyography was performed. Electromyography showed signs of myasthenia gravis, although the patient was asymptomatic. Thymectomy was performed, and thereafter the pathology report showed thymic Hodgkin lymphoma. Myasthenia gravis completely regressed after surgical treatment. The patient also received complementary chemotherapy afterward [8]. Lastly, in 2021, Yousfi et al reported a young female patient who presented with fatigue, weakness, and ptosis [9]. The diagnosis of seropositive myasthenia gravis was confirmed. Chest CT showed anterior mediastinal mass. Two months later, the patient presented with cervical lymph-adenopathy [9]. A biopsy was taken and showed the mixed cellularity type of Hodgkin lymphoma [9]. A positron-emission tomography scan showed an anterior mediastinal mass as well as supradiaphragmatic and subdiaphragmatic lymphadenopathy. Hodgkin lymphoma was staged as IIIA, since the patient was not experiencing constitutional symptoms. Unfortunately, the management and outcome were not discussed in the report [9].
The association between myasthenia gravis and Hodgkin lymphoma has not yet been clarified. This report represents a 24-year-old female patient who was diagnosed with a case of myasthenia gravis along with an incidental anterior mediastinal mass that was later confirmed to be Hodgkin lymphoma coexisting with thymoma.
Case Report
A 24-year-old female patient with a known case of vitiligo was admitted as a case of an incidental anterior mediastinal mass found on a routine chest radiograph (Figure 1). She had a 2-year history of diplopia, yet she did not seek medical advice before. The patient denied any history of muscle weakness, dysphagia, dyspnea, chest pain, night sweats, weight loss, or fever. The family history was insignificant, including no history of malignancy or myasthenia gravis. Physical examination was unremarkable, and lymph nodes were impalpable. Laboratory tests showed an elevated erythrocyte sedimentation rate of 44 mm/h and a C-reactive protein level of 1.6 mg/dL.
During admission, myasthenia gravis was diagnosed, which was confirmed by an electromyography test. Treatment began with oral pyridostigmine 60 mg administered 4 times per day, oral prednisolone 10 mg once daily, and oral azathioprine 100 mg once daily.
A chest CT scan with contrast was performed for further assessment of the mass (Figure 2). It showed a 3.7×5.4×7.2 cm homogenous anterior mediastinum soft tissue mass with a clear fat plane separating the mass from the vascular structure, which suggested a diagnosis of thymoma, with consideration of other differential diagnoses, such as lymphoma and teratoma.
The patient underwent median sternotomy, and complete dissection of the mass, mediastinal fat, and thymus gland was performed (Figure 3). Macroscopically, the specimen consisted of a tan-brown, rubbery, and lobulated thymus, weighing 93 g and measuring 9.0×8.0×3.5 cm. Sectioning showed a white-yellow lobulated cut surface with scattered cysts (Figure 4). An intra-thymic lymph node was identified and measured 1.2×1 cm.
Microscopically, hematoxylin and eosin–stained sections showed variably sized nodules surrounded by fibro-collagenous bands. Areas of high cellularity had scattered large atypical neoplastic cells. Several variants of these Reed-Sternberg like cells were present, including mononuclear Hodgkin cells, multinucleated Reed-Sternberg cells, lacunar cells, and mummified cells (Figure 5). They aggregated in confluent sheets in some areas (syncytial growth pattern). The background consisted of non-neoplastic polymorphic inflammatory cells with predominance of neutrophils, lymphocytes, plasma cells, and eosinophils (Figure 6). Thymic tissue was illustrated by the presence of large epithelial cells and prominent Hassall corpuscles. Some of these corpuscles were surrounded and infiltrated by Hodgkin cells (Figure 7). Additionally, the existence of numerous cysts lined by various types of epithelium and large medullary germinal centers (thymic hyperplasia) was confirmed (Figure 8). One lymph node showed Hodgkin lymphoma.
Immunohistochemistry stains revealed expression of CD15 and CD30 antigens by the Hodgkin cells, with the membranous and perinuclear (Glogi) staining patterns (Figure 9). However, they lacked CD45 expression and some of them showed weak membranous positivity for CD20. In the background, mixed reactivity of CD3 and CD5 was demonstrated (background T cells). Thymic epithelial cells showed membranous positivity for cytokeratin 19 (CK19) and focal membranous positivity for pan-cytokeratin (pan-CK) in an arborizing pattern (Figures 10, 11). They also showed nuclear positivity for protein 63 (P63) (Figure 12). The strong reactivity for CK19 and P63 with focal positivity for pan-CK denoted background thymoma. All immunohistochemical tests were performed on formalin-fixed (6–72 h) paraffin-embedded sections using the ultraview universal DAB detection system from FDA-approved Ventana.
The pathological diagnosis was classic Hodgkin lymphoma, nodular sclerosis subtype, syncytial variant, with background thymoma, and possible thymic follicular hyperplasia.
Clinically, it was staged as IIA Hodgkin lymphoma, according to the Cotswold classification. She was treated with 6 cycles of the ABVD chemotherapy protocol (doxorubicin, vinblastine, bleomycin, and dacarbazine). At present, after 4 years from surgical management, no evidence of Hodgkin lymphoma was detected during the follow-up. Regarding her ocular symptoms, after a latent period of 3.5 years, worsening of her symptoms was noticed in the postpartum period, which required dosage adjustment of her medications.
Discussion
Herein, we describe the case of a 24-year-old woman who had an incidental anterior mediastinal mass on a routine chest radiograph and 2-year history of diplopia. Myasthenia gravis was diagnosed, and the patient was treated with pyridostigmine, prednisolone, and azathioprine. Further assessment by a chest CT scan revealed a homogenous anterior mediastinum soft tissue mass, suggestive of thymoma, lymphoma, and teratoma. Surgical removal was performed, and the histopathology report revealed classic Hodgkin lymphoma, nodular sclerosis subtype, syncytial variant, with possible thymic follicular hyperplasia and background of thymoma. She received chemo-therapy, and presently, after 4 years of follow-up, no evidence of Hodgkin lymphoma was detected. However, she experienced worsening ocular symptoms in the postpartum period, requiring medication adjustment.
Myasthenia gravis prevalence is estimated to be 12.4 individuals per 100 000 globally [10]. Myasthenia gravis is defined as an autoimmune-mediated antibody disorder in which post-synaptic membrane components of the skeletal muscle fibers are attacked [11,12]. This attack leads to different symptoms, which vary among patients and include fluctuating ocular, bulbar, and limb skeletal muscle weakness [12]. This weakness can be generalized or specific to certain muscle groups, leading to various symptoms that depend on the involved muscles [12]. More than three-quarters of patients present initially with extra-ocular muscle weakness, such as diplopia and ptosis [12]. Bulbar muscle weakness can present initially in a much lower proportion of patients as dysphagia, flaccid dysarthria, and hoarseness [12]. In myasthenia gravis, limb weakness usually affects proximal muscles with upper limbs more than distal muscles and lower limbs [12].
Myasthenia gravis occurs in individuals who are genetically susceptible [4]. Precipitated factors include surgeries, drugs, infections, pregnancy, and immunization [12]. The pathophysiology of myasthenia gravis depends on the type of antibodies involved [10–12]. These antibodies act on nicotinic acetylcho-line receptors (n-AChRs), lipoprotein-related protein 4 (LPR4), and muscle-specific kinase to prevent binding of acetylcho-line to its receptor and inhibit n-AChR distribution and clustering [10–12]. These antibodies contribute to the diagnosis of myasthenia gravis, especially anti-nicotinic acetylcholine receptors [12]. Patients with generalized and pure ocular myasthenia gravis present with 80% and 50% positive anti-n-AChRs, respectively [12]. In patients who are seronegative for antibodies, electrophysiologic tests including single-fiber electromyography and repetitive nerve stimulation tests used to assess conduction delay in myasthenia gravis [12]. Myasthenia gravis manifests either as an autoimmune disease or as a paraneoplastic syndrome related to thymus tumors but is rarely reported with other tumors [13]. Pathological changes of the thymus are shown in most patients with n-AChR antibodies and in a portion of patients with LRP4 antibodies but rarely in muscle-specific kinase antibodies [13]. Early onset myasthenia gravis is frequently related to thymus lymphofollicular hyper-plasia, while the late onset exhibits age-related thymic involution [13]. In contrast, approximately 15% of patients have thymoma-associated myasthenia gravis [13]. These findings of thymic epithelial hyperplasia indicate the involvement of the thymus in the production of antibodies against the muscle receptors [13]. Management of symptomatic myasthenia gravis consists of acetylcholinesterase inhibitors; immunosuppressants, mainly glucocorticoids, can be added in case of persistent symptoms [12]. Intravenous immunoglobulin is beneficial in case of a myasthenic crisis [12]. Patients with early onset myasthenia gravis, thymoma associated with myasthenia gravis, or seronegative antibody are indicated for thymectomy [12].
Thymoma is an anterior mediastinum mass originating from the epithelial cells of the thymus, which can be discovered incidentally or through symptoms [1]. The presenting symptoms of thymoma are caused by paraneoplastic syndrome or are due to compression against adjacent organs leading to symptoms such as dyspnea, chest pain, or cough [1]. Myasthenia gravis is the most common paraneoplastic syndrome associated with thymoma; approximately 50% of patients with thymoma have myasthenia gravis [1]. However, patients with symptomatic myasthenia gravis are diagnosed earlier, so thymoma is discovered in less advanced stages [1]. The modalities used in diagnosis of thymoma are chest CT or MRI; however, the definitive diagnosis needs tissue that is obtained either by surgical resection or biopsy [1].
Hodgkin lymphoma is a monoclonal lymphoid neoplasm and rare malignancy with an estimated incidence rate of 2.6 cases per 100 000 individuals in the United States [2]. It is divided into 2 categories: classical Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma [2]. These 2 categories show differences in pathology and clinical presentation [12]. Classical Hodgkin lymphoma represents 95% of all Hodgkin lymphoma cases, which are further divided into 4 subtypes: lymphocyte-rich, lymphocyte-depleted, mixed cellularity, and nodular sclerosis Hodgkin lymphoma [2]. The precise cause of Hodgkin lymphoma is unknown. However, the risk of Hodgkin lymphoma increases in autoimmune diseases, immunosuppression, and Epstein-Barr virus infection [2]. Also, there is evidence of familial predisposition in Hodgkin lymphoma [2]. Patients with Hodgkin lymphoma often present with painless supradiaphragmatic lymphadenopathy and B symptoms including high fever, unexplained weight loss, and profuse night sweats [2]. If the enlargement of the mediastinal lymph nodes is significant, the mass effect can cause shortness of breath and chest pain [2]. In patients with extra-nodal disease, related clinical manifestations can occur [2].
Hodgkin lymphoma often involves the intrathoracic structure, mediastinal lymph nodes in particular [3]. Thymus gland involvement is reported, but the exact prevalence is unknown [3]. It is uncommon to have Hodgkin lymphoma of the thymus gland without lymphadenopathy [3]. Heron et al discovered 30% thymic enlargement in 50 patients with thoracic disease on CT scans [3]. All these patients with thymic enlargement at the time of diagnosis also had enlarged mediastinal lymph nodes [3]. Moreover, the frequency of thymic enlargement was higher (56%) in the series of Wernecke, in which there were only 6 cases of isolated thymic enlargement among 43 patients with newly diagnosed Hodgkin lymphoma [3]. Patients with thymic Hodgkin lymphoma are often asymptomatic until extrathymic disease develops [3].
Patients with myasthenia gravis show an increased risk of extra-thymic neoplasms, triple that of those who do not undergo thymectomy [14]. The only case reported of extrathymic Hodgkin lymphoma with myasthenia gravis was of a 44-year-old woman with ocular, bulbar, and limb muscle weakness that was diagnosed as myasthenia gravis with n-AChR antibody, which was managed with pyridostigmine [15]. In the same year, the patient presented with a left anterior neck mass, which was diagnosed after 3 years of her presentation of lymphoma with mixed cellularity. In this case, lymphoma was treated with 6 cycles of chemotherapy, and the patient noticed the resolution of her myasthenic symptoms, the n-AChR antibody level declined, and her myasthenia gravis medication was ceased [15].
To the best of our knowledge, only 5 cases have reported the association between myasthenia gravis and thymic Hodgkin lymphoma [5–9]. According to the reported cases, myasthenia gravis associated with thymic Hodgkin lymphoma is more common in females, as 4 of 5 cases were female patients, which is similar to the present case [5–9]. The presentation of myasthenia gravis in the previous cases varies, with most of them having muscle weakness in addition to bulbar or ocular symptoms, specifically ptosis, while our patient presented with just mild diplopia [5–7,9]. However, 1 patient was asymptomatic for myasthenia gravis and presented with thoracic symptoms [8].
Pathological reports of the previous cases revealed a domination of nodular sclerosing Hodgkin lymphoma, with 3 patients in addition to our own having this diagnosis [5–8]. Mixed cellularity Hodgkin lymphoma was observed in only 1 case [9]. Different stages of Hodgkin lymphoma were exhibited in the reported cases, in which 2 were stage II and the others were stage I and III [5–9].
Regarding the management of the cases, most of the patients underwent thymectomy followed by chemotherapy [6–9]. In 2 cases, chemotherapy was stopped due to adverse effects and was replaced with radiation therapy [6,7]. The first case, described in 1977, was the only case managed by radiation therapy in addition to thymectomy [5]. After thymectomy, remission of Hodgkin lymphoma as well as myasthenia gravis symptoms was noticed in all previous cases, in contrast to the present case, which had a latent period of 3.5 years, followed by the worsening of myasthenia gravis symptoms in the post-partum period [5–9].
Conclusions
The association between myasthenia gravis and Hodgkin lymphoma is still unclear. In contrast to this report, the complete remission of myasthenia gravis after the management of Hodgkin lymphoma in previous reports led to the conclusion that myasthenia gravis could be a complication or paraneo-plastic syndrome of Hodgkin lymphoma. It is suggested that research efforts be focused on examining this area in order to understand the relationship between Hodgkin lymphoma and myasthenia gravis.
Figures
References:
1... Robinson SP, Akhondi H, Thymoma. [Updated 2023 Jul 17]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK559291/
2... Kaseb H, Babiker HM, Hodgkin lymphoma. [Updated 2023 Jun 26]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK499969/
3... Wajdi K, Sameh M, Salma F, Primary Hodgkin’s disease of the thymus: Respir Med CME, 2010; 3(1); 15-17
4... Dresser L, Wlodarski R, Rezania K, Soliven B, Myasthenia gravis: Epidemiology, pathophysiology and clinical manifestations: J Clin Med, 2021; 10(11); 2235
5... Null JA, Livolsi VA, Glenn WW, Hodgkin’s disease of the thymus (granulomatous thymoma) and myasthenia gravis: A unique association: Am J Clin Pathol, 1977; 67(6); 521-25
6... Tranchant C, Racamier E, Warter JM, Seronegative myasthenia gravis and familial Hodgkin’s disease: Eur Neurol, 1993; 33(1); 17-19
7... Ferrò MT, Riccardi T, Montanelli A, Myasthenia gravis remission and anti-AChR ab reduction after immunosuppressive and anti-neoplastic therapy in a patient with thymic Hodgkin’s disease: J Neurol, 2006; 253(9); 1241-42
8... Ayadi-Kaddour A, Mlika M, Kilani T, El Mezni F, A primary mediastinal Hodgkin’s lymphoma with asymptomatic myasthenia gravis: A rare association: Pathologica, 2008; 100(3); 170-72
9... Yousfi J, Bensalek F, Zahlane M, Myasthenia gravis revealing Hodgkin’s lymphoma: Eur J Case Rep Intern Med, 2021; 8(3); 002375
10... Salari N, Fatahi B, Bartina Y, Global prevalence of myasthenia gravis and the effectiveness of common drugs in its treatment: A systematic review and meta-analysis: J Transl Med, 2021; 19(1); 516
11... Phillips WD, Vincent A, Pathogenesis of myasthenia gravis: Update on disease types, models, and mechanisms: F1000Res, 2016; 5; F1000 Faculty Rev-1513
12... Beloor Suresh A, Asuncion RMD, Myasthenia gravis. [Updated 2023 Aug 8]: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK559331/
13... Melzer N, Ruck T, Fuhr P, Clinical features, pathogenesis, and treatment of myasthenia gravis: A supplement to the Guidelines of the German Neurological Society: J Neurol, 2016; 263(8); 1473-94
14... Papatestas AE, Osserman KE, Kark AE, The relationship between thymus and oncogenesis. A study of the incidence of non thymic malignancy in myasthenia gravis: Br J Cancer, 1971; 25(4); 635-45
15... Abrey LE, Association of myasthenia gravis with extrathymic Hodgkin’s lymphoma: Complete resolution of myasthenic symptoms following antineo-plastic therapy: Neurology, 1995; 45(5); 1019
Figures
In Press
17 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943370
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250