05 January 2024: Articles 
Life-Threatening Electrical Storm Following Liver Transplantation: A Case Report
Unknown etiology
David Richer Araujo Coelho1ABEF*, Rogerio Oliveira da Luz1AE, Samanta Teixeira Basto2E, Mauro Rogério De Barros Wanderley Júnior3E, Claudia Cristina Tavares de Sousa2E, Elke Reis Fagundes de Carvalho1E, Eduardo de Sousa Martins Fernandes2E, Anderson Brito-Azevedo4AEDOI: 10.12659/AJCR.941932
Am J Case Rep 2024; 25:e941932
Abstract
BACKGROUND: Electrical storm is a rare but potentially life-threatening syndrome characterized by recurrent ventricular arrhythmias. Liver transplant recipients are at increased risk of developing electrical storms due to conditions that prolong QT intervals, such as cirrhotic cardiomyopathy. However, limited information exists on electrical storms in this specific population. This case report presents a patient who experienced 13 cardiac arrests during ventricular fibrillation following liver transplantation.
CASE REPORT: A 61-year-old woman with a medical history of diabetes, obesity, and cirrhosis due to non-alcoholic fatty liver disease underwent liver transplantation using a deceased donor’s liver. Following the procedure, she developed a deterioration in her respiratory function, necessitating orotracheal intubation. Approximately 21 hours post-surgery, she experienced cardiac arrest during ventricular fibrillation, which was rapidly reversed with electrical defibrillation. However, the patient entered a state of electrical storm. Management involved antiarrhythmic medications and temporary transvenous cardiac pacing. She remained stable for 40 hours, but a dislodgment of the device triggered another episode of ventricular fibrillation, leading to her death.
CONCLUSIONS: This case report highlights the clinical presentation and challenges in managing electrical storms in liver transplant recipients. We hypothesize that cirrhotic cardiomyopathy could be the cause of her recurrent ventricular arrhythmias. Further studies are needed to better understand the underlying mechanisms and risk factors of this life-threatening syndrome in this population, which may enhance risk stratification and enable earlier intervention.
Keywords: Arrhythmias, Cardiac, case reports, Intensive Care Units, Liver Transplantation, Ventricular Fibrillation
Background
Electrical storm (ES) is a rare but potentially life-threatening syndrome characterized by sustained, recurrent, and symptomatic ventricular arrhythmias [1]. Since its initial identification, numerous authors have described the frequency and duration of episodes that qualify as clinically significant events [2]. The most commonly accepted definition for ES is “3 or more episodes of ventricular tachycardia or ventricular fibrillation (VF) within 24 hours” [3].
The incidence of ES is estimated to be from 6% to 14% in patients with implantable cardioverter-defibrillators (ICDs) and has a high mortality rate [4,5]. The presentation varies from mild symptoms such as palpitations and chest discomfort to more severe outcomes such as syncope, cardiac arrest, and sudden death [6]. The underlying mechanisms are complex and multifactorial, with myocardial ischemia, sympathetic activation, electrolyte imbalances, and drug toxicity among the many contributing factors [7].
Despite advances in treatment options, ES remains a clinical challenge, often presenting as a medical emergency [8]. Treatment strategies include antiarrhythmic medications, electrical cardioversion, and temporary transvenous cardiac pacing (TTCP) [9,10]. However, optimal management depends on the underlying etiology, the severity of the arrhythmias, and the patient’s overall clinical status [11].
ES has been reported in various clinical settings, such as after cardiac surgery and in patients with structural heart disease [12–14]. Nevertheless, its occurrence in the context of liver transplantation (LT) has not been well documented, especially in patients without ICDs. In this case report, we present a case of ES in a patient undergoing LT and discuss the potential trigger, clinical presentation, diagnosis, and management of this potentially lethal arrhythmia.
Case Report
A 61-year-old woman with a medical history of diabetes, obesity, and cirrhosis due to non-alcoholic fatty liver disease was admitted to the hospital for spontaneous bacterial peritonitis. Both diabetes and cirrhosis were diagnosed a year previously, and she underwent multiple paracentesis procedures for relief over the prior 3 months. Her liver disease severity was indicated by a Child-Turcotte-Pugh score of C and a Model for End-Stage Liver Disease (MELD) score of 31.
The patient had no family history of cardiac conditions or personal history of smoking, alcohol consumption, or drug use. Her medications included metformin, furosemide, and lactu-lose. On examination, her vital signs were stable, with a temperature of 36.9°C, blood pressure of 110/80 mmHg, heart rate of 82 beats per minute, SpO2 of 98%, and a respiratory rate of 17 breaths per minute. She exhibited noticeable pallor (3+/4+) and jaundice (2+/4+). Physical findings included bilateral edema (2+/4+) and pronounced ascites. Heart and lung sounds were normal.
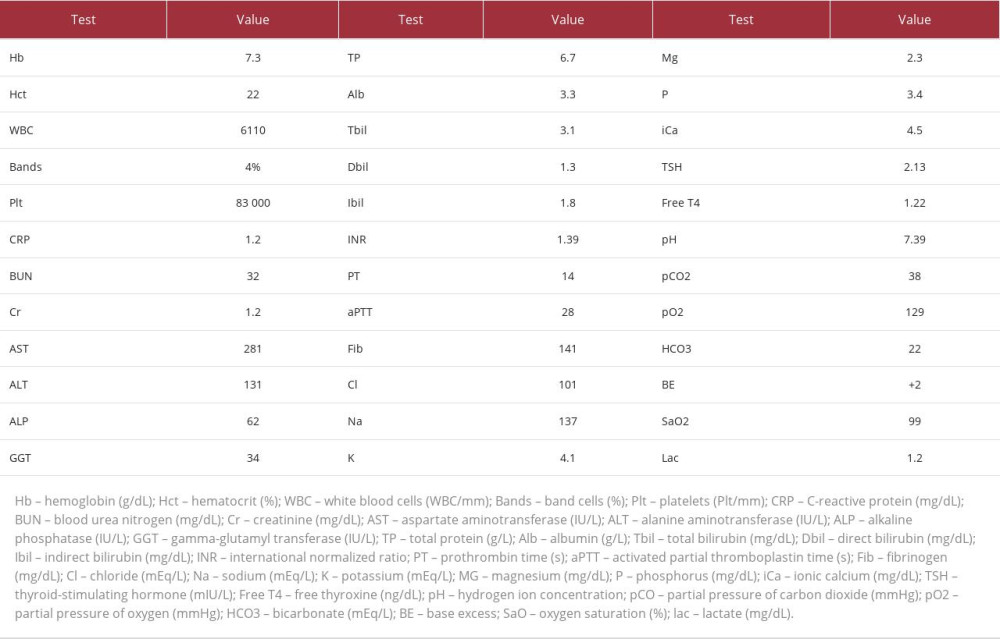
Laboratory results on admission (detailed in Table 1) showed normocytic normochromic anemia, thrombocytopenia, elevated liver function tests, decreased albumin levels, increased bilirubin, and a prolonged international normalized ratio (INR). Thyroid function tests were within normal limits. A preoperative electrocardiogram (ECG) revealed normal sinus rhythm and QT interval; the echocardiogram was unremarkable.
Upon admission, the patient was administered 2 units of packed red blood cells and regular albumin infusions. A therapeutic paracentesis was conducted, which drained 4860 ml of turbid yellowish fluid. Initial antibiotic treatment with meropenem was started but was later switched to ceftriaxone after fluid cultures identified a multi-susceptible
By the third day of hospitalization, her renal function deteriorated (creatinine: 2.6 mg/dL and BUN: 78 mg/dL), and she became oligoanuric. Despite the use of conservative methods, such as diuretics, lactulose, and albumin, her renal function did not improve. Our approach was to initiate continuous renal replacement therapy (CRRT) preoperatively as a treatment option to stabilize and compensate the patient for surgery.
On the sixth day of hospitalization, following stabilization of her renal function and resolution of the spontaneous bacterial peritonitis, LT was performed from a deceased 54-year-old male donor. The donor had a history of social alcohol use and died from a hemorrhagic stroke.
The surgery was performed using the conventional technique with splenic ligation and choledochal-choledochal anastomosis and was completed successfully without any complications. There were no signs of venous thrombosis or temporary portacaval shunt. The total ischemia time was 3 hours and 13 minutes, cold ischemia time was 2 hours and 46 minutes, and warm ischemia time was 27 minutes.
During the procedure, the patient received 3000 ml of crystalloids, and multiple blood products were transfused due to blood dyscrasia, including 20 units of cryoprecipitates, 4 units of packed red blood cells, 6 units of fresh frozen plasma, and 12 units of platelets. Additionally, 9000 ml of ascitic fluid was drained. Surgical prophylaxis included piperacillin-tazobactam and fluconazole.
Immediately postoperatively, the patient was admitted to the Intensive Care Unit (ICU), receiving low-dose vasopressor support, oxygen via a nasal catheter, and CRRT. However, approximately 6 hours after LT, her respiratory pattern worsened. Despite the administration of corticosteroids, she developed refractory bronchospasm and respiratory acidosis. Non-invasive ventilation was ineffective, and she required orotracheal intubation. Post-intubation, arterial blood gases normalized. A subsequent computerized tomography scan confirmed the graft’s integrity and showed no signs of hepatic complications.
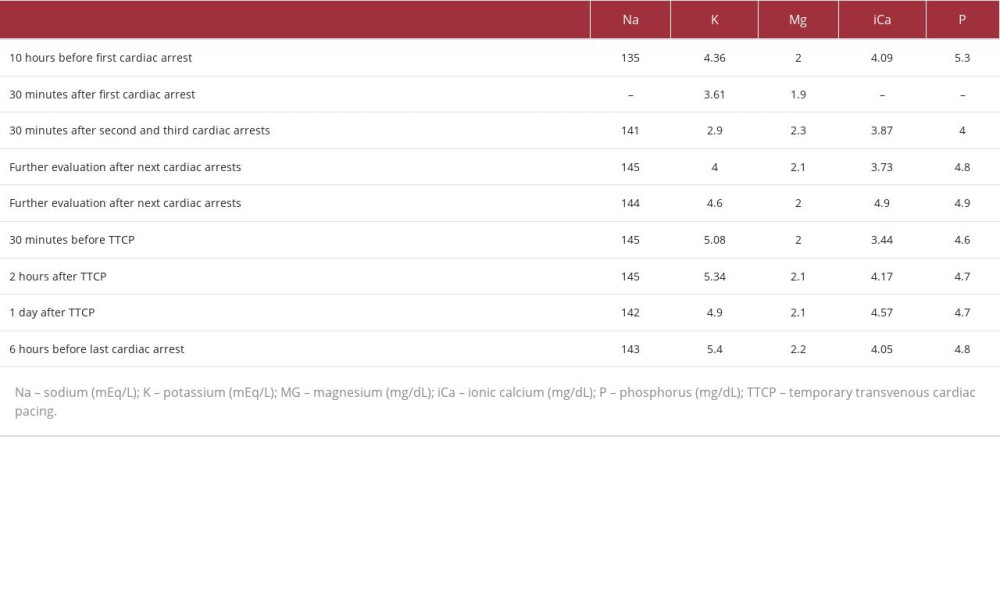
The following day, on the seventh day of hospitalization, within 21 hours after LT, the patient experienced a cardiac arrest (CA) during VF while undergoing CRRT without any volume removal by ultrafiltration. The CA resolved after 30 seconds without the need for defibrillation. The patient’s potassium and magnesium levels were 3.61 mEq/L and 1.9 mg/dL (Table 2), respectively, and arterial blood gas showed adequate partial pressure of oxygen without any signs of metabolic or respiratory acidosis. She received post-CA care, was kept on mechanical ventilation, and was sedated with midazolam and fentanyl.
After this initial event, the patient experienced 2 more consecutive CAs during VF, each lasting less than a minute, that were quickly reversed by prompt electrical defibrillation. Her heart rhythm returned to atrial fibrillation, and amiodarone was administered. A post-second event potassium measurement showed a level of 2.9 mEq/L (Table 2), which was immediately corrected with intravenous potassium chloride in a polarizing solution.
An ES was suspected based on the patient’s presentation. A 12-lead ECG confirmed a corrected QT prolongation (QTc) of 710 ms (as shown in Figure 1). Administration of amiodarone was maintained. Although indicated for ES, beta-blockers were not administered due to the patient’s hemodynamic instability requiring high doses of vasopressors.
Another echocardiogram was performed, and revealed several abnormalities, including mild mitral and tricuspid valve re-flux, an increased left atrium, diffuse hypokinesis of the left ventricle walls, and global systolic dysfunction ranging from mild to moderate. Grade I diastolic dysfunction was also present. In addition, the echocardiogram demonstrated a pulmonary artery systolic pressure of 51 mmHg and an inferior vena cava measuring 2.3 cm. No signs of pulmonary thromboembolism, cardiac tamponade, or pneumothorax were observed.
Over the following 8 hours, the patient experienced 10 other episodes of CA during VF, each lasting less than a minute and reverting spontaneously to the same heart rhythm depicted in Figure 1, with QT prolongation. However, 2 episodes progressed to asystoles lasting 4 and 18 minutes. Potassium and magnesium levels remained normal, as shown in Table 2. Her thyroid-stimulating hormone (TSH) and free T4 levels were also normal, being 0.8 mIU/L and 1.3 ng/dL, respectively.
Upon consulting the electrophysiology team, it was recommended to discontinue using amiodarone and initiate a continuous infusion of lidocaine through a pump. Furthermore, the team advised discontinuing any other medication that could potentially cause long QT syndrome, such as fluconazole. A TTCP was inserted, with a heart rate set between 100 and 110 beats per minute to shorten the QT interval. Additional parameters were a sensitivity of 7 mV and a voltage of 8 V.
After configuring the TTCP, the patient remained hemodynamically stable with no further episodes of VF but no improvement of QTc prolongation. The initial plan was to remove the TTCP if the QT interval was less than 500 ms. However, after approximately 40 hours without further episodes of VF, a dislodgment of the TTCP occurred, which precipitated another episode. This episode progressed into asystole, culminating in the patient’s death.
Discussion
In this case report, we describe a 61-year-old woman who developed ES following LT. While ES is a recognized complication in various cardiac conditions, its prevalence and management in the context of LT remains limited [15,16].
ES is typically precipitated by underlying structural heart disease. The most common etiologies include nonischemic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, sarcoidosis, amyloidosis, Chagas’ disease, and Brugada syndrome [17]. Despite our patient’s ECG and echocardiogram at admission showing no abnormalities, we hypothesize that cirrhotic cardiomyopathy (CCM) could be the cause of her ES. Notably, patients with CCM are generally asymptomatic with near-normal cardiac function, and the condition manifests under periods of cardiovascular stress, such as LT [18,19].
CCM is characterized by cardiac dysfunction in patients with cirrhosis who do not have other known cardiac diseases [20]. It is defined by an impaired cardiac response to stress, a hyperdynamic circulatory state, both diastolic and systolic dysfunctions, and QT prolongation [21,22]. Additionally, other complications of CCM are pulmonary congestion and arrhythmia [23,24]. After transplantation, our patient’s pulmonary function deteriorated, and she experienced ES. Her ECG and echocardiogram after the CAs revealed a QTc prolongation of 710 ms, left bundle branch block, and both mild to moderate global systolic and grade I diastolic dysfunctions. These postoperative findings, which were absent before surgery, underscore CCM as a probable trigger for her ES.
Beyond CCM, liver transplant recipients are vulnerable to several causes that can lead to the development of ES. Immunosuppressants, such as tacrolimus and cyclosporine, are frequently administered post-transplantation to prevent graft rejection [25]. These drugs have been implicated in QT prolongation [26]. However, our patient did not receive immunosuppressants due to her brief postoperative duration and thrombocytopenia [27]. Another medication for QT prolongation is fluconazole [28]. Although the patient received a single dose of this antifungal immediately after surgery as prophylaxis, its role in her ES appears minimal [29].
Other potential causes are electrolyte abnormalities, particularly hypokalemia, hypomagnesemia, and hypocalcemia, which increase the risk of ventricular arrhythmias [30]. Our patient presented with an isolated episode of hypokalemia with a serum K level of 2.9 mEq/L after her second CA, which was promptly corrected. Subsequent CAs could not be attributable to hypokalemia or hypomagnesemia, as her K and Mg levels remained within the normal range. Hypocalcemia is recognized for its potential to induce QT prolongation [31,32]. While our patient was regularly administered intravenous calcium gluconate as a corrective measure, her ionic calcium levels predominantly remained below the threshold of 4.5 mg/dL. This sustained hypocalcemia could be another contributor to her QT prolongation and the subsequent ES.
Myocardial infarction has been documented as a potential trigger for ES [33,34]. It is worth noting, though, that our patient did not present with acute onset chest pain, and her troponin curve did not show elevations, ruling out the possibility of a myocardial infarction. Thyrotoxicosis could be another trigger of ES, but TSH and free T4 levels were within normal limits throughout her hospitalization [35,36]. Furthermore, patients undergoing LT can experience an acute release of inflammatory cytokines days after surgery, potentially leading to cardiac dysfunction and arrhythmias due to over-activation of the autonomic nervous system [37–39]. Nevertheless, our patient’s rapid onset of pulmonary congestion and CA, occurring within 6 hours and 21 hours post-LT, respectively, suggests a limited role of surgery-related stress in the development of her ES and aligns more closely with the characteristics of CCM [23,24].
To our knowledge, this is the second documented case of ES in a liver transplant recipient without an ICD. Schmidt and Muir (2003) reported a case of an 18-year-old woman with Wilson’s disease who underwent LT and experienced recurrent VF in a total of 27 separate VF arrests [40]. Similarly, her VF episodes responded to cardioversion, but sinus rhythm was not maintained for prolonged periods. The authors also administered amiodarone and lidocaine, as well as TTCP. One difference between our case and the one reported by Schmidt and Muir (2003) was the administration of 5 mg of metoprolol in the latter case, which resulted in immediate resolution of VF with no further recurrences [40]. Our patient’s hemodynamic instability precluded such an approach [41]. Instead, we relied on a combination of antiarrhythmic medications and TTCP [9]. Although QTc prolongation did not improve, our patient remained stable for 40 hours with no further CAs until a TTCP dislodgment occurred, leading to her death.
In summary, while CCM is our primary hypothesis for the development of ES in this case, there are other important causes of arrhythmias in liver transplant recipients, including drug toxicities, electrolyte imbalances, myocardial infarction, thyrotoxicosis, and activation of the sympathetic nervous system due to surgery-related stress [24,26,28,30,33,35,38,39]. It is crucial to closely monitor the potential risk of ES in liver transplant recipients in the ICU and implement appropriate intervention strategies to prevent adverse outcomes in this population.
Conclusions
Despite the unfortunate outcome, our case contributes to the limited literature on ES in liver transplant recipients, highlighting the importance of early recognition and management of this potentially life-threatening complication. We hypothesize that CCM could be the cause of ES in this patient. Healthcare providers should be aware of the potential risk factors for the development of ES in patients who undergo LT and be prepared to intervene promptly. Further research is needed to better understand the pathophysiology of this rare cardiovascular complication in this population to enhance risk stratification and enable earlier intervention.
References:
1.. Sagone A, Electrical storm: Incidence, prognosis and therapy: J Atr Fibrillation, 2015; 8; 1150
2.. Barnay C, Taieb J, Morice R, [Rhythmic storms]: Ann Cardiol Angéiologie, 2007; 56; 183-87 [in French]
3.. Gao D, Sapp JL, Electrical storm: Definitions, clinical importance, and treatment: Curr Opin Cardiol, 2013; 28; 72-79
4.. Arya A, Haghjoo M, Dehghani MR, Prevalence and predictors of electrical storm in patients with implantable cardioverter-defibrillator: Am J Cardiol, 2006; 97; 389-92
5.. Guerra F, Shkoza M, Scappini L, Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: A meta-analysis: Europace, 2014; 16(3); 347-53
6.. Zarse M, Hasan F, Khan A, [Electrical storm: Recognition and management]: Herzschrittmachertherapie Elektrophysiologie, 2020; 31; 55-63 [in German]
7.. Dyer S, Mogni B, Gottlieb M, Electrical storm: A focused review for the emergency physician: Am J Emerg Med, 2020; 38; 1481-87
8.. Elsokkari I, Tsuji Y, Sapp JL, Nattel S, Recent insights into mechanisms and clinical approaches to electrical storm: Can J Cardiol, 2022; 38(4); 439-53
9.. Guarracini F, Bonvicini E, Zanon S, Emergency management of electrical storm: A practical overview: Med Kaunas Lith, 2023; 59; 405
10.. Jentzer JC, Noseworthy PA, Kashou AH, Multidisciplinary critical care management of electrical storm: JACC state-of-the-art review: J Am Coll Cardiol, 2023; 81; 2189-206
11.. Elsokkari I, Sapp JL, Electrical storm: Prognosis and management: Prog Cardiovasc Dis, 2021; 66; 70-79
12.. Rehorn MR, Black-Maier E, Loungani R, Electrical storm in patients with left ventricular assist devices: Risk factors, incidence, and impact on survival: Heart Rhythm, 2021; 18; 1263-71
13.. Nademanee K, Taylor R, Bailey WE, Treating electrical storm: Sympathetic blockade versus advanced cardiac life support-guided therapy: Circulation, 2000; 102; 742-47
14.. Sielatycki P, Chlabicz M, Sawicki R, A 39-year-old woman with ventricular electrical storm treated with emergency cardiac defibrillation followed by multidisciplinary management: Am J Case Rep, 2022; 23; e935710
15.. Muser D, Liang JJ, Santangeli P, Electrical storm in patients with implantable cardioverter-defibrillators: A practical overview: J Innov Card Rhythm Manag, 2017; 8; 2853-61
16.. Bárzaga FJT, Clavijo PC, Sánchez MD, [Electrical storm in patients with implantable cardioverter-defibrillator.]: Arch Cardiol Mex, 2008; 78; 68-78 [in Spanish]
17.. Dusi V, Angelini F, Gravinese C, Electrical storm management in structural heart disease: Eur Heart J Suppl, 2023; 25(Suppl. C); C242-C48
18.. Lyssy LA, Soos MP, Cirrhotic cardiomyopathy: StatPearls [Internet], 2023, Treasure Island (FL), StatPearls Publishing [cited 2023 Oct 29]
19.. Liu H, Jayakumar S, Traboulsi M, Cirrhotic cardiomyopathy: Implications for liver transplantation: Liver Transplant, 2017; 23; 826-35
20.. Baik SK, Fouad TR, Lee SS, Cirrhotic cardiomyopathy: Orphanet J Rare Dis, 2007; 2; 15
21.. Izzy MJ, VanWagner LB, Current concepts of cirrhotic cardiomyopathy: Clin Liver Dis, 2021; 25; 471-81
22.. Carvalho MVH, Kroll PC, Kroll RTM, Cirrhotic cardiomyopathy: The liver affects the heart: Braz J Med Biol Res, 2019; 52; e7809
23.. Milani A, Zaccaria R, Bombardieri G, Cirrhotic cardiomyopathy: Dig Liver Dis, 2007; 39; 507-15
24.. Liu S-H, Lo L-W, Chou Y-H, Evidence of ventricular arrhythmogenicity and cardiac sympathetic hyperinnervation in early cirrhotic cardiomyopathy: Front Physiol, 2021; 12; 719883
25.. Di Maira T, Little EC, Berenguer M, Immunosuppression in liver transplant: Best Pract Res Clin Gastroenterol, 2020; 46–47; 101681
26.. Ikitimur B, Cosansu K, Karadag B, Long-term impact of different immunosuppressive drugs on QT and PR intervals in renal transplant patients: Ann Noninvasive Electrocardiol, 2014; 20; 426-32
27.. Moini M, Schilsky ML, Tichy EM, Review on immunosuppression in liver transplantation: World J Hepatol, 2015; 7; 1355-68
28.. Wang J, Wang G, Quan X, Fluconazole-induced long QT syndrome via impaired human ether-a-go-go-related gene (hERG) protein trafficking in rabbits: Europace, 2017; 19; 1244-49
29.. Evans JDW, Morris PJ, Knight SR, Antifungal prophylaxis in liver transplantation: A systematic review and network meta-analysis: Am J Transplant, 2014; 14; 2765-76
30.. Laslett DB, Cooper JM, Greenberg RM, Electrolyte abnormalities in patients presenting with ventricular arrhythmia (from the LYTE-VT study): Am J Cardiol, 2020; 129; 36-4
31.. Tang JKK, Rabkin SW, Hypocalcemia-induced QT interval prolongation: Cardiology, 2022; 147; 191-95
32.. Eryol NK, Colak R, Ozdoğru I, Effects of calcium treatment on QT interval and QT dispersion in hypocalcemia: Am J Cardiol, 2003; 91; 750-52
33.. Ohsawa S, Isono H, Ojima E, Electrical storm 11 days after acute myocardial infarction: A case report: J Med Case Reports, 2019; 13; 346
34.. Liu B, Xie B, Chen X, A successful case of electrical storm rescue after acute myocardial infarction: BMC Cardiovasc Disord, 2022; 22; 537
35.. Erdogan HI, Gul EE, Gok H, Therapy-resistant ventricular tachycardia caused by amiodarone-induced thyrotoxicosis: A case report of electrical storm: Am J Emerg Med, 2012; 30; 2092.e5-7
36.. Marketou ME, Simantirakis EN, Manios EG, Electrical storm due to amiodarone induced thyrotoxicosis in a young adult with dilated cardiomyopathy: thyroidectomy as the treatment of choice: Pacing Clin Electrophysiol PACE, 2001; 24; 1827-28
37.. Kaltenmeier C, Wang R, Popp B, Role of immuno-inflammatory signals in liver ischemia-reperfusion injury: Cells, 2022; 11; 2222
38.. Finnerty CC, Mabvuure NT, Ali A, The surgically induced stress response: J Parenter Enteral Nutr, 2013; 37; 21S-29S
39.. Shen MJ, Zipes DP, Role of the autonomic nervous system in modulating cardiac arrhythmias: Circ Res, 2014; 114; 1004-21
40.. Schmidt TD, Muir AJ, A case of electrical storm in a liver transplant patient: Transplant Proc, 2003; 35; 1437-38
41.. Wołowiec Ł, Grześk G, Osiak J, Beta-blockers in cardiac arrhythmias – clinical pharmacologist’s point of view: Front Pharmacol, 2022; 13; 1043714
In Press
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943803
18 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943467
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.943376
19 Mar 2024 : Case report 
Am J Case Rep In Press; DOI: 10.12659/AJCR.942853
Most Viewed Current Articles
07 Mar 2024 : Case report 
DOI :10.12659/AJCR.943133
Am J Case Rep 2024; 25:e943133
10 Jan 2022 : Case report 
DOI :10.12659/AJCR.935263
Am J Case Rep 2022; 23:e935263
19 Jul 2022 : Case report 
DOI :10.12659/AJCR.936128
Am J Case Rep 2022; 23:e936128
23 Feb 2022 : Case report 
DOI :10.12659/AJCR.935250
Am J Case Rep 2022; 23:e935250